-
Abstract: Embryonic stem cells possess fascinating capacity of self-renewal and developmental potential, leading to significant progress in understanding the molecular basis of pluripotency, disease modeling, and reprogramming technology. Recently, 2-cell–like embryonic stem cells (ESCs) and expanded potential stem cells or extended pluripotent stem cells (EPSCs) generated from early-cleavage embryos display some features of totipotent embryos. These cell lines provide valuable in vitro models to study underlying principles of totipotency, cell plasticity, and lineage segregation. In this review, we summarize the current progress in this filed and highlight the application potentials of these cells in the future.
-
Key words:
- 2C-like ESC /
- EPSC /
- MERVL /
- Totipotency
-
1. Introduction
Embryonic stem cells (ESCs), derived from the inner cell mass (ICM) of a blastocyst, possess the ability of indefinite self-renewal and to form all the cell types of three germ layers (Smith, 2001). This fascinating capacity attracts research interest, and significant progress has been achieved in understanding the molecular basis of pluripotency, disease modeling, and reprogramming technology. For instance, identification of key factors governing pluripotency network leads to the invention of induced pluripotent stem cell (iPSC) technology (Takahashi and Yamanaka, 2006), a powerful reprogramming strategy that can be used to generate patient-specific pluripotent stem cells without ethical concerns. Strikingly, several recent studies have suggested that a small population of ESCs are capable of transiently entering a state resembling 2-cell (2C)–stage embryos (Macfarlan et al., 2012). These cells display better reprogramming ability through somatic cell nuclear transfer (Ishiuchi et al., 2015). Besides, recent studies utilize 2C-like ESCs as an in vitro model to understand the onset of zygotic genome activation (ZGA) (De Iaco et al., 2017; Hendrickson et al., 2017; Whiddon et al., 2017; Eckersley-Maslin et al., 2019). More recently, a more stable cell line which is named expanded potential stem cells or extended pluripotent stem cells (EPSCs) with expanded developmental potential to form both embryonic and extraembryonic (ExEm) lineages is established from 4-cell–stage or 8-cell–stage embryos (Yang et al., 2017a, 2017b, 2019; Gao et al., 2019). Blastocyst-like structures can be generated from EPSCs, providing a unique in vitro model for understanding the early embryogenesis (Li et al., 2019). These studies highlight the versatile applications of these cell lines in understanding the basic principles of embryogenesis and somatic cell reprogramming (Fig. 1).
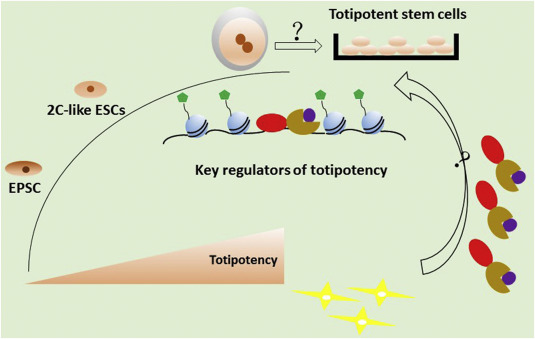
 Fig. 1. 2C-like ESCs and EPSCs provide in vitro models to identify key regulators involved in the establishment of totipotency, which will facilitate the capture and establishment of totipotency in vitro. 2C, 2-cell; ESCs, embryonic stem cells; EPSCs, expanded potential stem cells or extended pluripotent stem cells.
Fig. 1. 2C-like ESCs and EPSCs provide in vitro models to identify key regulators involved in the establishment of totipotency, which will facilitate the capture and establishment of totipotency in vitro. 2C, 2-cell; ESCs, embryonic stem cells; EPSCs, expanded potential stem cells or extended pluripotent stem cells.In addition, the recent breakthrough in the development of low-input assays enables researchers to depict the genome-wide epigenetic and transcriptional profiles of early embryos (Xu and Xie, 2018). The underlying principle of totipotency, cell plasticity, and lineage segregation is beginning to emerge. Taken together, the recent advancement opens up a door for understanding the molecular basis of totipotency and raises an interesting question: whether totipotency can be captured and maintained in vitro.
2. Embryonic development and totipotency
Mouse embryo development undergoes sequential processes including zygote, 2-cell, 4-cell, 8-cell, morulae, blastocyst, implantation, and postimplantation development. During the first four days of embryonic development, a single fertilized egg undergoes a series of cell divisions and morphological changes, forming an epithelial vesicle filled with a flowing liquid and covering a dense group of cells, the ICM. When developing into late blastocysts, the outer epithelial cells form trophectoderm (TE), and then TE differentiates into ExEm trophectoderm and trophoblastic giant cells, with the former involved in the formation of placenta. Upon development into the expanded blastocyst, ICM cells have differentiated into two distinct cell populations, the primitive endoderm (PE) near the blastocyst and the epiblast (EPI) away from the blastocyst. EPI is pluripotent with the capacity to form all of the original germ layers of the fetus and their ExEm tissues, while PE primarily forms the endoderm of the ExEm yolk sac.
Mouse zygotes and 2C blastomeres possess the capacity to give rise to an entire organism, the ability defined as totipotency (Tarkowski, 1959). In other mammalian species including rabbits, sheep, cattle, and monkeys, totipotency extends to 4-cell– to 8-cell–stage embryos (Moore et al., 1968; Willadsen, 1981; Johnson et al., 1995; Mitalipov et al., 2002). In the subsequent cleavage divisions, embryonic cells gradually lose totipotency and differentiate into two lineages—ICM and TE. Uncovering the molecular basis of totipotency regulation and the first cell fate segregation is a fundamental question of developmental biology. During early embryonic development in mammals, blastomeres within embryos are morphologically indistinguishable before the 8-cell stage (Rossant and Tam, 2009). However, recent studies showed that molecular heterogeneity is already present at 4-cell to 8-cell stages associated with the fate segregation of ICM and TE (Piotrowska-Nitsche et al., 2005; Torres-Padilla et al., 2007; Plachta et al., 2011; Tabansky et al., 2013; Goolam et al., 2016; White et al., 2016).
Arginine methyltransferase 1 (CARM1) is distributed asymmetrically among 4-cell blastomeres (Torres-Padilla et al., 2007). CARM1 upregulates levels of histone H3 arginine 26 methylation (H3R26me), and the blastomeres with high H3R26me develop preferentially to ICM (Torres-Padilla et al., 2007). Recently, studies have shown that CARM1 can increase the number of DNA-binding sites associated with Oct4/Sox2 and also promote the expression of their downstream target genes (e.g., Sox21) (Plachta et al., 2011; Goolam et al., 2016; White et al., 2016). At the same time, PRDM14 and CARM1 function synergistically to increase H3R26me levels in the blastomeres of 4-cell embryos (Burton et al., 2013). Therefore, in the 4-cell stage, elevated protein levels of the CARM1/PRDM14-Oct4/Sox2-Sox21 axis contribute to the ICM cell fate decision.
Even with the aforementioned studies, the molecular mechanism underlying the heterogeneity of the 4-cell blastomeres remains to be elusive. A recent study revealed that heterogeneity arises even at the 2C stage (Shi et al., 2015). The expression level of a long noncoding RNA, LincGET, differs between the blastomeres of the late 2C embryos, and the differences continuously increase in the 4-cell embryos. The aforementioned results inspired the present studies to further explore the role of LincGET during the first cell fate determination in the early embryos.
3. Identification of 2C-like ESCs
ESCs are not homogeneous but contain subpopulations with distinct gene expression profiles. For example, Gata6, a critical transcription factor for ExEm development, is expressed in Nanog-low ESCs (Singh et al., 2007). An interesting possibility was proposed that heterogeneous ESC subpopulations may harbor different development potential. Indeed, although ESCs are capable of forming EPI and ExEm PE cells at single-cell level, some cells display only EPI potential (Canham et al., 2010). Intriguingly, ESCs contain subpopulations with expanded developmental potential. For instance, Hex-positive population of ESCs is capable of contributing to both embryonic and ExEm lineages (Morgani et al., 2013). Recently, a rare ESC subpopulation marked by the reactivation of endogenous retrovirus MERVL has been identified (Macfarlan et al., 2012). Transcriptome analysis indicated that this ESC subpopulation highly expresses genes restricted to 2C embryos and lacks the expression of Oct4, Sox2, and Nanog. Thus, these cells are named 2C-like ESCs. 2C-like ESCs are unstable, and almost every cell is capable of transiently entering 2C-like state.
Retrotransposon MERVL acts as an alternative promoter to drive proximal gene transcription in 2C-like ESCs. Among these genes reactivated in 2C-like ESCs, the Zscan4 gene cluster is essential for the long-term maintenance of genomic stability and telomeres in ESCs (Zalzman et al., 2010). The Zscan4 gene cluster comprises six paralogs with high sequence similarities (Falco et al., 2007). Zscan4d is exclusively transcribed in late 2C embryos, while Zscan4c is predominantly transcribed in ESCs (Falco et al., 2007; Zalzman et al., 2010). Zscan4c expression is also dynamic and transient in mouse ESCs. Repression of Zscan4c in ESCs does not cause immediate response but results in karyotype abnormality and loss of indefinitely self-renewal ability by 7–8 passages (Zalzman et al., 2010). MERVL is reactivated during the first transcription wave of ZGA, whereas Zscan4d is transcribed in late 2C embryos (Falco et al., 2007). More recently, Zscan4c is found to be an activator of MERVL, indicating that Zscan4 gene cluster can activate the 2C state (Zhang et al., 2019). Unlike their expression pattern in vivo, Zscan4-positive ESCs seem equivalent to MERVL-positive ESCs at least based on gene expression features (Eckersley-Maslin et al., 2016).
2C-like ESCs acquire an expanded ability to contribute to both the embryonic and ExEm lineages. Upon injection into morulae embryo, descendants of 2C-like ESCs are capable of contributing to both the TE and ICM of blastocysts, whereas ESCs contribute only to the ICM (Macfarlan et al., 2012). However, this study could not exclude the possibility that 2C-like ESCs are a heterogeneous mixture of lineage-biased cells rather than a single 2C-like cell with expanded developmental potential.
4. Chromatin features of 2C-like ESCs
2C-like ESCs possess highly relaxed chromatin architecture, similar to 2C embryos. Genome-wide demethylation takes place upon ESCs entering 2C-like state, including gene bodies, promoters, and repeat elements such as MERVL element (Eckersley-Maslin et al., 2016). This global loss of DNA methylation seems to be a consequence of entering 2C-like state rather than a cause, as DNMT-triple knockout or naïve 2i medium culture could not upregulate the MERVL-LTR transcription network (Eckersley-Maslin et al., 2016). Strikingly, genome-wide DNA methylation level is restored when ESCs exit from 2C-like state. Transient burst of Zscan4 in 2C-like ESCs is responsible for this genome-wide DNA demethylation via degradation of Uhrf1 and Dnmt1 (Dan et al., 2017).
In addition to DNA methylation, significant changes of histone modifications occur upon ESCs entering 2C-like state, accompanied by transient activation of repetitive regions and ZGA-associated genes (Akiyama et al., 2015; Ko, 2016). At heterochromatin regions, active mark H3K27ac is greatly increased, while repressive marks H3K9me2 and DNA methylation are still abundant (Akiyama et al., 2015). This unique signature may be associated with the quick repression of heterochromatin region when ESCs cycle out of a 2C-like state. As for genes reactivated in 2C-like ESCs, the same trend of increased level of H3K27ac is observed (Akiyama et al., 2015). But unlike the heterochromatin region, DNA methylation level is also reduced in these so-called facultative heterochromatin regions, indicating the different regulatory mechanism (Akiyama et al., 2015). Zscan4c is capable of forming complex with epigenetic modifiers, indicating its role in epigenetic regulation (Storm et al., 2014; Akiyama et al., 2015; Dan et al., 2017; Zhang et al., 2019). Besides DNA methylation and histone modifications, 2C-like ESCs display high histone mobility and increased level of chromatin accessibility (Boskovic et al., 2014; Ishiuchi et al., 2015).
5. Regulation of 2C-like ESCs
Only a rare fraction of ESCs could enter 2C-like state at a given time point. There are several gatekeepers preventing ESCs from reopening the 2C-like transcriptome. Several groups focused on the regulatory mechanism of MERVL and Zscan4 in ESCs recently. These studies provide insights into the regulatory mechanism of the 2C-like transcriptome in ESCs.
MERVL is activated among the first transcription wave during ZGA and functions as retroviral promoter to drive gene transcription in 2C-embryos and 2C-like ESCs (Macfarlan et al., 2012). DNA methylation is recognized as a critical mechanism to silence endogenous transposable elements. Strikingly, DNA methylation does not account for the derepression of MERVL in 2C-like ESCs (Matsui et al., 2010; Karimi et al., 2011; Eckersley-Maslin et al., 2016). G9a-dependent H3K9me2 plays an essential role in maintaining the repression states of MERVL (Maksakova et al., 2013). Furthermore, although deletion of H3K9 methyltransferase, Kap1, is capable of activation of MERVL, this action is a consequence of indirect effect as MERVL is not marked with H3K9me3, nor efficiently bound by Kap1 (Maksakova et al., 2013). Another repressor, Chd5, inhibits MERVL by regulating H3K27me3 and H3.1/H3.2 function (Hayashi et al., 2016). In addition, loss of CAF-1, a histone chaperone, significantly increases the incidence of ESCs entering 2C-like state (Ishiuchi et al., 2015).
Noncoding RNA miR34 also plays an important role in restricting the developmental potential of ESCs (Choi et al., 2017). In a previous study, miR34 has been identified as a key barrier for iPSC reprogramming (Choi et al., 2011). Following this finding, the authors further showed that miR34-deficient iPSCs and ESCs display an expanded developmental potential resembling 2C-like ESCs (Choi et al., 2017). Furthermore, the authors demonstrated the expanded cell fate potential does not result from ExEm-biased subpopulation, as a single miR34−/− ESCs could contribute to both ICM and TE in chimeric blastocysts (Choi et al., 2017). miR34 represses MERVL induction via direct downregulation of Gata2 (Choi et al., 2017).
Thus far, although multiple players have been identified to silence the 2C-like transcriptome in ESCs, the upstream regulatory mechanism of 2C-like ESCs remains to be elusive. Tbx3 was found to function as an activator of 2C-like state (Dan et al., 2013). It was shown that Tbx3 can activate Zscan4 and Tcstv1/3 at subtelomere regions through downregulation of global DNA methylation level (Dan et al., 2013). More recently, several studies highlighted Dux family of transcription factors as key drivers for ESCs entering 2C-like state (De Iaco et al., 2017; Hendrickson et al., 2017; Whiddon et al., 2017). The discovery of Dux gene family is from an irrelevant study of a muscle disorder termed facioscapulohumeral dystrophy (Gabriels et al., 1999; Geng et al., 2012). Abnormal activation of Dux results in activation of genes associated with cleavage-stage embryos in muscle cells. Mouse genome contains a large number of Dux family genes, many of which are nested in tandem array macrosatellites (Leidenroth et al., 2012). Mouse Dux is expressed exclusively at early 2C embryos, before the activation of MERVL at middle 2C embryo stage (De Iaco et al., 2017; Hendrickson et al., 2017; Whiddon et al., 2017). In ESCs, induced Dux expression efficiently increased the efficiency of MERVL-positive cells from 10% up to 74% within 24 h (De Iaco et al., 2017; Hendrickson et al., 2017; Whiddon et al., 2017). In addition, depletion of Dux completely blocks the activation of MERVL (De Iaco et al., 2017; Hendrickson et al., 2017; Whiddon et al., 2017). Activation of Dux in mouse ESCs leads to upregulation of 2C-specific transposons and genes, such as MERVL, Zscan4 gene family, and Tcstv1/2 (De Iaco et al., 2017; Hendrickson et al., 2017; Whiddon et al., 2017). Furthermore, Dux functions as a pioneer factor to open the specific locus and provokes the activation of downstream targets (De Iaco et al., 2017; Hendrickson et al., 2017). Identifying the upstream regulators of Dux is essential to understand the induction of 2C-like state in cultures and provides clues for studying the mechanism of ZGA. Recent studies indicated that developmental pluripotency–associated 2 (Dppa2) and developmental pluripotency–associated 4 (Dppa4) can increase Dux expression level by performing a screen in ESCs (De Iaco et al., 2019; Eckersley-Maslin et al., 2019; Yan et al., 2019). Dppa2 and Dppa4 are capable of activating 2C genes via direct regulation of Dux (De Iaco et al., 2019; Eckersley-Maslin et al., 2019). These findings indicate that Dppa2 and Dppa4 are important to ZGA and the establishment of 2C-like state. Loss of Dppa3 in embryos leads to the failure of MERVL transcript upregulation in 2C stage (Huang et al., 2017). Interestingly, Dux was found to be important but not essential to ZGA progression (Chen and Zhang, 2019; Guo et al., 2019). In embryonic development, Dppa2 and Dppa4 are maternally expressed, which are before ZGA. Dux is transiently expressed in the early stage of 2-cells, and then gradually decreases, and is no longer expressed in the late stage of the 2-cells. Zsacn4 gene cluster is expressed in the middle and late stages of 2-cells, and the peak expression level is reached in middle 2C stage (Falco et al., 2007). However, whether these factors activate in 2C-like state in the same order as embryonic development remains unclear. Dppa2/4 can promote Dux expression, and Dux can also bind to the Dppa2 promoter (Eckersley-Maslin et al., 2019). In addition, Dux is capable of activating Zscan4 in a direct manner, while Zscan4 cannot bind to the Dux locus (Hendrickson et al., 2017; Eckersley-Maslin et al., 2019). In addition, Zscan4c overexpression upregulates the expression of Dux and Dppa2/4 in ESCs, suggesting an indirect regulation manner of Zscan4c (Zhang et al., 2019). Dux, Dppa2/4, and Zscan4 all can activate the expression of MERVL and 2-cell/4-cell genes in ESCs (Zhang et al., 2019). These findings indicate a positive feedback loop among these factors to reinforce the 2C-like state in ESCs. But the exact order and the initial factor of this positive feedback loop are still unclear.
Following these findings, several inhibitors were found to impede the 2C embryos and 2C-like ESCs. Through a CRISPR-Cas9 screen under Dux-induction of 2C-like ESCs, researchers found that Myc acts as an inhibitor of initial phase during 2C-like transition through promoting the expression of downregulated genes in 2C-like state, while Dnmt1 inhibits the activation of upregulated genes in 2C-like state (Fu et al., 2019). H3.3 represses the expression of Dux and related ZGA genes by directly binding to the promotor region and gene body of Dux (Tian et al., 2020). Small ubiquitin-like modifier (Sumo) E3 ligase protein inhibitor of activated STAT 4 (PIAS4) is an inhibitor of zygotic transcriptional program likely through sumoylating multiple regulation factors in zygotic transcription including Dppa2 (Yan et al., 2019). The authors showed that the sumoylation of Dppa2 by PIAS4 turns it from an activator to a potent inhibitor of ZGA. They also provided a possible relationship between Dppa2 and Dppa4 that Dppa2 functions as the activator of ZGA and Dppa4 can enhance the function of Dppa2 (Yan et al., 2019).
In view of the important function of Zscan4 in 2C-like ESCs, there are several studies focused on the modulation of Zscan4 (Dan et al., 2014; Storm et al., 2014; Nakai-Futatsugi and Niwa, 2016; Yang et al., 2016; Zhang et al., 2016; Bai et al., 2019). These studies also provide important insights into the upstream regulatory mechanism. Zscan4 gene cluster locates in the subtelomere region and is enriched with heterochromatic epigenetic marks (Dan et al., 2014). Interruption of repressive marks significantly reactivates Zscan4 (Dan et al., 2013, 2014; Yang et al., 2016). In mouse ESCs, Rif1 suppresses Zscan4 via stabilizing H3K9me3 at subtelomere heterochromatins (Dan et al., 2014).
Using MERVL or Zscan4 as reporter of 2C-like ESCs, multiple players have been shown to modulate the 2C-like transcriptome in ESCs (Table 1). However, whether these effectors are specific or essential for activation of 2C-like state needs to be further determined. In addition, it is still not clear about the molecular details before ESCs converting into 2C-like state. In response to telomere attrition and DNA damage, 2C-specific gene Zscan4 is efficiently reactivated, indicating that entry into 2C-like state may not be stochastic (Storm et al., 2014; Nakai-Futatsugi and Niwa, 2016). A possible explanation is that in 2C-like state, the errors accumulated in long-term culture could be modified. Surely, ESCs benefit from the transient entrance into 2C-like state. As previously mentioned, transient activation of 2C-specific genesZscan4 and Tcstv1/2 associated with telomere extension, which is critical for the genomic stability of ESCs in the long-term culture. More importantly, back into 2C-like state is critical for the maintenance of pluripotency in ESCs. 2C-depleted ESCs display bias toward mesoderm and ectoderm differentiation (Macfarlan et al., 2012).
Table 1. Known activators and repressors of 2-cell like state.Function Factor Description Reference Activator MERVL Retroviral promoters to drive gene transcription in 2C-like ESCs Macfarlan etal. (2012) Zscan4 2C-like state marker, activating MERVL Zhang etal. (2019) Dux Key drivers for ESCs entering 2C-like state De Iaco etal. (2017), Hendrickson etal. (2017), Whiddon et al. (2017). Tbx3 Downregulation of global methylation and derepression of Zsacn4 and Tcstv1/3 Dan etal. (2013) Dppa2/4 Upregulation of Dux Eckersley-Maslin etal. (2019) Repressor G9a G9a-dependent H3K9me2 maintains the repression state of MERVL Maksakova etal. (2013) Kap1 Indirect repression of MERVL Maksakova etal. (2013) Chd5 Inhibiting MERVL by regulating H3K27me3 and H3.1/H3.2 function Hayashi etal. (2016) miR34 Repressing MERVL induction via direct downregulation of Gata2 Choi etal. (2017) H3.3 Dux repressor Tian etal. (2020) PIAS4 Sumoylation of Dppa2 by PIAS4 turns it from an activator to a potent inhibitor of ZGA Yan etal. (2019) Dnmt1 Inhibiting the activation of upregulated genes in the 2C-like state Fu etal. (2019) Myc Promoting the expression of downregulated genes in the 2C-like state Fu etal. (2019) CAF-1 Downregulation of CAF-1 can induce mouse 2C-like cells Ishiuchi etal. (2015) 2C, 2-cell; ESCs, embryonic stem cells; ZGA, zygotic genome activation. 6. The molecular trajectory of 2C-like ESC emergence
Dissecting the process of the spontaneous transition from ESCs toward 2C-like state is important to understand the mechanism of totipotency acquisition in culture. By performing the single-cell expression profiling, two recent studies identified the intermediate states in the process of 2C-like transition (Rodriguez-Terrones et al., 2018; Fu et al., 2019). By analyzing 93 representative genes, researchers from the laboratory of Maria-Elena Torres-Padilla uncovered a sequential model of transcription changes (Rodriguez-Terrones et al., 2018). Before the acquisition of a 2C-like identity, cells exhibited gradually upregulated Zscan4 expression levels. Based on the levels of Zscan4 transcripts, three intermediate states could be identified, Zscan4low, Zscan4middle, and Zscan4high. Specific transcription factors and chromatin modifiers displayed significant expression changes among these Zscan4+ intermediates. Along with the transcription changes, the chromatin landscape of Zscan4+ cells exhibited intermediate to those of ESCs and 2C-like cells.
By performing single-cell RNA-seq, Fu et al. provided a more comprehensive molecular roadmap during the transition process (Fu et al., 2019). To enrich 2C-like cells, the authors overexpressed Dux in ESCs (Fu et al., 2019). Transcriptome of Dux-induced 2C-like cells is very similar to that of spontaneous 2C-like cells. Besides, Dux-induced 2C-like state is transient, resembling spontaneous 2C-like cells (Fu et al., 2019). Using the Dux-induced transition system, the authors discovered that reprogramming from pluripotency to a 2C-like state is a two-step process (Fu et al., 2019). In the initial stage, the major characteristics are downregulation of pluripotent genes (Fu et al., 2019). Myc impedes this stage by maintaining the expression of pluripotent genes (Fu et al., 2019). Activation of 2C genes and repeats takes place in a late stage (Fu et al., 2019). The inefficient transition during this stage can be alleviated by suppression of Dnmt1 (Fu et al., 2019).
7. EPSCs
More recently, a significant breakthrough has been made in the derivation of EPSCs from 4-cell or 8-cell embryos. These EPSCs display extended pluripotency, which means they have the ability to generate ExEm tissues, including the trophoblast lineages contributing to the development of placental, as well as embryonic tissues (Yang et al., 2017a,b). A small-molecule cocktail was finally identified, to block signaling pathways affecting TE/ICM segregation (Yang et al., 2017a,b).
To date, researchers designed two different cocktails of chemical molecules. One is comprised of hLIF, CHIR99021, DiM, and MiH (LCDM), the other contains PD0325901, JNK Inhibitor VIII, SB203580, A-419259, XAV939, and CHIR99021, and as few as three inhibitors (CHAX) can also take effect (Table 2). These molecules block the pathways related to trophoblastic differentiation. Mitogen-activated protein kinases are blocked by PD0325901, JNK Inhibitor VIII, and SB203580 (Azzolin et al., 2014; Kapoor et al., 2014; Shao et al., 2014). A-419259 is selected to block Src kinase, which blocks the partly arrested morulae and blastocysts (Wilson et al., 2002). XAV939 is selected to repress TNKS1/2, family members of Parp, and to maintain the level of AXIN, which regulates the concentration of β-catenin and Yap1 destruction complex. XAV939 also inhibits Yap1 activity through angiomotin 14, associated with its potential role in the lineage segregation of ICM and TE (Huang et al., 2009; Azzolin et al., 2014; Wang et al., 2015). Furthermore, it was found that pluripotent stem cells from mice, porcine, as well as human can be converted to EPSCs to gain the ability to give rise to trophoblast stem cell–like cells under in vitro culture conditions (Gao et al., 2019). It was reported that DiM can inhibit the activity of G protein–coupled receptors and MiH can repress PARP-1 (Pfaff et al., 1995; Alano et al., 2006). EPSCs can also be derived by LCDM with the chimeric ability to give rise to the embryonic and ExEm lineages at the single-cell level. It is worth noting that LCDM-derived single human EPSC can also give rise to the interspecies human-mouse conceptuses.
Table 2. Two different cocktails of chemical molecules to block the pathways related to trophoblastic differentiation.Cocktail Inhibitor Target pathway Function of the pathway Species Reference CHAX CHIR99021 GSK-3β Energy metabolism, neuronal cell development, and body pattern formation Mus musculus, Sus scrofa, or Homo sapiens Martello etal. (2012) PD0325901 Mitogen-activated protein kinases Proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis Pearson etal. (2001) JNK Inhibitor VIII SB203580 A-419259 Src kinase Partly arrested morula and affected trophectoderm and PrE Wilson etal. (2002) XAV939 Poly(ADP-ribose) polymerase family Detect and initiate an immediate cellular response to metabolic-, chemical-, or radiation-induced single-strand DNA breaks (SSB) Herceg and Wang (2001) LCDM hLIF Interleukin 6classcytokine The growth promotion and cell differentiation of different types of target cells Mus musculus or Homo sapiens Hu etal. (2007) CHIR99021 GSK-3β Energy metabolism, neuronal cell development, and body pattern formation Martello etal. (2012) DiM G protein–coupled receptors Detectmoleculesoutside thecelland activate internalsignal transductionpathways and, ultimately, cellular responses Pfaff etal. (1995) MiH Poly(ADP-ribose) polymerase I Required for the induction ofICAM-1 gene expression by smooth muscle cells, in response to TNF Alano et al. (2006) TNF, tumor necrosis factor. EPSCs show different genome-wide epigenetic modifications from conventional pluripotent stem cells and the high levels of DNA methyltransferases including Dnmt1, Dnmt3a, and Dnmt3b, as well as demethylases such as Tet1, Tet2, and Tdg resembling 4-cell to 8-cell blastomeres (Deng et al., 2014). Meanwhile, the increased number of bivalent genes is closely linked with the extended pluripotency of EPSCs (Marks et al., 2012). Besides, EPSCs have a similar gene expression pattern resembling 2C-stage embryos. Taken together, these results reveal that EPSCs have distinctive molecular characteristics that differ from known pluripotent stem cell types.
EPSCs generated by CHAX or LCDM have been proved that single cell has developmental capacity to form Em and ExEm lineages. Therefore, EPSCs are a useful in vitro system to understand the molecular mechanisms of cell fate determination. In addition, owing to the provision of a large number of cells, there is great potential of EPSCs in disease model establishment, drug screening, and regenerative medicine in the future.
8. Implications for embryogenesis
After fertilization, sequential events are initiated to reprogram terminal differentiated gametes into totipotent embryos. Parental epigenetic marks are quickly erased to re-establish zygotic epigenome. In this regard, similar extensive epigenetic remodeling occurs upon ESCs entering into 2C-like state, including global DNA demethylation and acquisition of active histone marks. The drastic epigenetic change leads to genome-wide open chromatin states in totipotent embryos and 2C-like ESCs. It is recognized that this unique chromatin feature is associated with ZGA. A possible explanation is that the increased chromatin open state enables the transcription factors to access to their targets. Besides, open chromatin state may permit the derepression of genes, retrotransposons, and other repetitive sequences wrapped in heterochromatins. Recent studies have shown that the link between epigenetic changes and transcription activation is much more complex than we think. Overexpression of Dux, recently reported as a key driver of ZGA, markedly increases the regions gaining ATAC signals in 2C-like ESCs, resembling that in 2C embryos (Hendrickson et al., 2017). By performing chromatin immunoprecipitation sequencing (ChIP-seq), the authors further showed that at least 37% of the regions gaining ATAC signals are bound by Dux. The data indicate that Dux regulates the activation of its targets via opening the chromatin structures. It is noteworthy that a number of 2C-associated genes are independent of Dux regulation, indicating the existence of other pioneer factors (De Iaco et al., 2017). Furthermore, maternal inherited factors are suggested to serve as pioneer factors to initiate ZGA. 2C-like ESCs may provide an in vitro model to test this hypothesis.
Transcription of early-cleavage embryos is highly dynamic, including two major transcription waves. The first wave corresponds to ZGA, begins at late 1-cell stage, and peaks at 2-cell to 4-cell embryo stage (Hamatani et al., 2004). The second wave, named mid-preimplantation gene activation (MGA), increases from 4-cell to 8-cell embryo (Hamatani et al., 2004). This stage-specific expression pattern fulfills the requirement of a developing embryo, leading to the gradual restriction of cell plasticity and the first cell lineage segregation (Hamatani et al., 2004). However, the function of these genes and the regulatory mechanism of the step-wise pattern is largely unknown because of the scarcity of samples. Future studies of transcription network of 2C-like ESC and EPSCs from 4-cell or 8-cell embryo may provide clues for the underlying mechanism exploration.
9. Conclusion
Totipotency refers to the capacity of a single cell to form an entire organism. In mice, only zygotes and 2C blastomeres possess this amazing ability and developmental potency is gradually restricted in the subsequent 4-cell to 8-cell embryos (Tarkowski, 1959; Rossant, 1976). Although 2C-like ESCs and EPSCs possess expanded developmental potential, whether these cells are totipotent or just with higher plasticity is still in debate. Contribution to ExEm and embryonic tissue when injected into embryos is not a stringent criterion of totipotency. It is the lack of evidence that these cells are capable of self-organization and forming an entire functional embryo. A more detailed comparison of epigenetic, transcriptome, functional features between expanded potential stem cells and totipotent early embryos is needed in the future. Although there is still a long way ahead to uncover the mystery of totipotency, the current studies surely provide insights into the basic principle of development, enhance the reprograming strategy and potentially benefit the clinical applications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31970758) and National Key R&D Program of China (2016YFA0102200, 2017YFA0103301, 2018YFC1004001).
-
Fig. 1. 2C-like ESCs and EPSCs provide in vitro models to identify key regulators involved in the establishment of totipotency, which will facilitate the capture and establishment of totipotency in vitro. 2C, 2-cell; ESCs, embryonic stem cells; EPSCs, expanded potential stem cells or extended pluripotent stem cells.
Table 1. Known activators and repressors of 2-cell like state.
Function Factor Description Reference Activator MERVL Retroviral promoters to drive gene transcription in 2C-like ESCs Macfarlan etal. (2012) Zscan4 2C-like state marker, activating MERVL Zhang etal. (2019) Dux Key drivers for ESCs entering 2C-like state De Iaco etal. (2017), Hendrickson etal. (2017), Whiddon et al. (2017). Tbx3 Downregulation of global methylation and derepression of Zsacn4 and Tcstv1/3 Dan etal. (2013) Dppa2/4 Upregulation of Dux Eckersley-Maslin etal. (2019) Repressor G9a G9a-dependent H3K9me2 maintains the repression state of MERVL Maksakova etal. (2013) Kap1 Indirect repression of MERVL Maksakova etal. (2013) Chd5 Inhibiting MERVL by regulating H3K27me3 and H3.1/H3.2 function Hayashi etal. (2016) miR34 Repressing MERVL induction via direct downregulation of Gata2 Choi etal. (2017) H3.3 Dux repressor Tian etal. (2020) PIAS4 Sumoylation of Dppa2 by PIAS4 turns it from an activator to a potent inhibitor of ZGA Yan etal. (2019) Dnmt1 Inhibiting the activation of upregulated genes in the 2C-like state Fu etal. (2019) Myc Promoting the expression of downregulated genes in the 2C-like state Fu etal. (2019) CAF-1 Downregulation of CAF-1 can induce mouse 2C-like cells Ishiuchi etal. (2015) 2C, 2-cell; ESCs, embryonic stem cells; ZGA, zygotic genome activation. Table 2. Two different cocktails of chemical molecules to block the pathways related to trophoblastic differentiation.
Cocktail Inhibitor Target pathway Function of the pathway Species Reference CHAX CHIR99021 GSK-3β Energy metabolism, neuronal cell development, and body pattern formation Mus musculus, Sus scrofa, or Homo sapiens Martello etal. (2012) PD0325901 Mitogen-activated protein kinases Proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis Pearson etal. (2001) JNK Inhibitor VIII SB203580 A-419259 Src kinase Partly arrested morula and affected trophectoderm and PrE Wilson etal. (2002) XAV939 Poly(ADP-ribose) polymerase family Detect and initiate an immediate cellular response to metabolic-, chemical-, or radiation-induced single-strand DNA breaks (SSB) Herceg and Wang (2001) LCDM hLIF Interleukin 6classcytokine The growth promotion and cell differentiation of different types of target cells Mus musculus or Homo sapiens Hu etal. (2007) CHIR99021 GSK-3β Energy metabolism, neuronal cell development, and body pattern formation Martello etal. (2012) DiM G protein–coupled receptors Detectmoleculesoutside thecelland activate internalsignal transductionpathways and, ultimately, cellular responses Pfaff etal. (1995) MiH Poly(ADP-ribose) polymerase I Required for the induction ofICAM-1 gene expression by smooth muscle cells, in response to TNF Alano et al. (2006) TNF, tumor necrosis factor. -
[1] Akiyama, T., Xin, L., Oda, M., Sharov, A.A., Amano, M., Piao, Y., Cadet, J.S., Dudekula, D.B., Qian, Y., Wang, W., Ko, S.B., Ko, M.S., 2015. Transient bursts of Zscan4 expression are accompanied by the rapid derepression of heterochromatin in mouse embryonic stem cells. DNA Res. 22, 307-318. [2] Alano, C.C., Kauppinen, T.M., Valls, A.V., Swanson, R.A., 2006. Minocycline inhibits poly(adp-ribose) polymerase-1 at nanomolar concentrations. Proc. Nat.l Acad. Sci. U. S. A. 103, 9685-9690. [3] Azzolin, L., Panciera, T., Soligo, S., Enzo, E., Bicciato, S., Dupont, S., Bresolin, S., Frasson, C., Basso, G., Guzzardo, V., Fassina, A., Cordenonsi, M., Piccolo, S., 2014. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158, 157-170. [4] Bai, L.G., Yang, L., Zhao, C.Q., Song, L.S., Liu, X.F., Bai, C.L., Su, G.H., Wei, Z.Y., Li, G.P., 2019. Histone demethylase UTX is an essential factor for zygotic genome activation and regulates Zscan4 expression in mouse embryos. Int. J. Biol. Sci. 15, 2363-2372. [5] Boskovic, A., Eid, A., Pontabry, J., Ishiuchi, T., Spiegelhalter, C., Raghu Ram, E.V., Meshorer, E., Torres-Padilla, M.E., 2014. Higher chromatin mobility supports totipotency and precedes pluripotency in vivo. Genes Dev. 28, 1042-1047. [6] Burton, A., Muller, J., Tu, S.J., Padilla-Longoria, P., Guccione, E., Torres-Padilla, M.E., 2013. Single-cell profiling of epigenetic modifiers identifies PRDM14 as an inducer of cell fate in the mammalian embryo. Cell Rep. 5, 687-701. [7] Canham, M.A., Sharov, A.A., Ko, M.S.H., Brickman, J.M., 2010. Functional heterogeneity of embryonic stem cells revealed through translational amplification of an early endodermal transcript. PLoS Biol. 8, e1000379. [8] Chen, Z., Zhang, Y., 2019. Loss of Dux causes minor defects in zygotic genome activation and is compatible with mouse development. Nat. Genet. 51, 947-951. [9] Choi, Y.J., Lin, C.P., Ho, J.J., He, X., Okada, N., Bu, P., Zhong, Y., Kim, S.Y., Bennett, M.J., Chen, C., Ozturk, A., Hicks, G.G., Hannon, G.J., He, L., 2011. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol. 13, 1353-1360. [10] Choi, Y.J., Lin, C.P., Risso, D., Chen, S., Kim, T.A., Tan, M.H., Li, J.B., Wu, Y., Chen, C., Xuan, Z., Macfarlan, T., Peng, W., Lloyd, K.C., Kim, S.Y., Speed, T.P., He, L., 2017. Deficiency of microrna miR-34a expands cell fate potential in pluripotent stem cells. Science 355, eaag1927. [11] Dan, J., Li, M., Yang, J., Li, J., Okuka, M., Ye, X., Liu, L., 2013. Roles for Tbx3 in regulation of two-cell state and telomere elongation in mouse ES cells. Sci. Rep. 3, 3492. [12] Dan, J., Rousseau, P., Hardikar, S., Veland, N., Wong, J., Autexier, C., Chen, T., 2017. Zscan4 inhibits maintenance DNA methylation to facilitate telomere elongation in mouse embryonic stem cells. Cell Rep. 20, 1936-1949. [13] Dan, J.M., Liu, Y.F., Liu, N., Chiourea, M., Okuka, M., Wu, T., Ye, X.Y., Mou, C.L., Wang, L., Wang, L.L., Yin, Y., Yuan, J.H., Zuo, B.F., Wang, F., Li, Z.G., Pan, X.H., Yin, Z.N., Chen, L.Y., Keefe, D.L., Gagos, S., Xiao, A., Liu, L., 2014. Rif1 maintains telomere length homeostasis of ESCs by mediating heterochromatin silencing. Dev. Cell 29, 7-19. [14] De Iaco, A., Coudray, A., Duc, J., Trono, D., 2019. Dppa2 and Dppa4 are necessary to establish a 2C-like state in mouse embryonic stem cells. EMBO Rep. 20, e47382. [15] De Iaco, A., Planet, E., Coluccio, A., Verp, S., Duc, J., Trono, D., 2017. Dux-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 49, 941-945. [16] Deng, Q., Ramskold, D., Reinius, B., Sandberg, R., 2014. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193-196. [17] Eckersley-Maslin, M., Alda-Catalinas, C., Blotenburg, M., Kreibich, E., Krueger, C., Reik, W., 2019. Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. Genes Dev. 33, 194-208. [18] Eckersley-Maslin, M.A., Svensson, V., Krueger, C., Stubbs, T.M., Giehr, P., Krueger, F., Miragaia, R.J., Kyriakopoulos, C., Berrens, R.V., Milagre, I., Walter, J., Teichmann, S.A., Reik, W., 2016. MERVL/Zscan4 network activation results in transient genome-wide DNA demethylation of mESCs. Cell Rep. 17, 179-192. [19] Falco, G., Lee, S.L., Stanghellini, I., Bassey, U.C., Hamatani, T., Ko, M.S., 2007. Zscan4: A novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev. Biol. 307, 539-550. [20] Fu, X., Wu, X., Djekidel, M.N., Zhang, Y., 2019. Myc and Dnmt1 impede the pluripotent to totipotent state transition in embryonic stem cells. Nat. Cell Biol. 21, 835-844. [21] Gabriels, J., Beckers, M.C., Ding, H., De Vriese, A., Plaisance, S., van der Maarel, S.M., Padberg, G.W., Frants, R.R., Hewitt, J.E., Collen, D., Belayew, A., 1999. Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene 236, 25-32. [22] Gao, X., Nowak-Imialek, M., Chen, X., Chen, D., Herrmann, D., Ruan, D., Chen, A.C.H., Eckersley-Maslin, M.A., Ahmad, S., Lee, Y.L., Kobayashi, T., Ryan, D., Zhong, J., Zhu, J., Wu, J., Lan, G., Petkov, S., Yang, J., Antunes, L., Campos, L.S., Fu, B., Wang, S., Yong, Y., Wang, X., Xue, S.G., Ge, L., Liu, Z., Huang, Y., Nie, T., Li, P., Wu, D., Pei, D., Zhang, Y., Lu, L., Yang, F., Kimber, S.J., Reik, W., Zou, X., Shang, Z., Lai, L., Surani, A., Tam, P.P.L., Ahmed, A., Yeung, W.S.B., Teichmann, S.A., Niemann, H., Liu, P., 2019. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 21, 687-699. [23] Geng, L.N., Yao, Z., Snider, L., Fong, A.P., Cech, J.N., Young, J.M., van der Maarel, S.M., Ruzzo, W.L., Gentleman, R.C., Tawil, R., Tapscott, S.J., 2012. Dux4 activates germline genes, retroelements, and immune mediators: Implications for facioscapulohumeral dystrophy. Dev. Cell 22, 38-51. [24] Goolam, M., Scialdone, A., Graham, S.J.L., Macaulay, I.C., Jedrusik, A., Hupalowska, A., Voet, T., Marioni, J.C., Zernicka-Goetz, M., 2016. Heterogeneity in Oct4 and Sox2 targets biases cell fate in 4-cell mouse embryos. Cell 165, 61-74. [25] Guo, M., Zhang, Y., Zhou, J., Bi, Y., Xu, J., Xu, C., Kou, X., Zhao, Y., Li, Y., Tu, Z., Liu, K., Lin, J., Yang, P., Gao, S., Wang, Y., 2019. Precise temporal regulation of Dux is important for embryo development. Cell Res. 29, 956-959. [26] Hamatani, T., Carter, M.G., Sharov, A.A., Ko, M.S., 2004. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell 6, 117-131. [27] Hayashi, M., Maehara, K., Harada, A., Semba, Y., Kudo, K., Takahashi, H., Oki, S., Meno, C., Ichiyanagi, K., Akashi, K., Ohkawa, Y., 2016. Chd5 regulates MuERV-L/MERVL expression in mouse embryonic stem cells via H3k27me3 modification and histone H3.1/H3.2. J. Cell. Biochem. 117, 780-792. [28] Hendrickson, P.G., Dorais, J.A., Grow, E.J., Whiddon, J.L., Lim, J.W., Wike, C.L., Weaver, B.D., Pflueger, C., Emery, B.R., Wilcox, A.L., Nix, D.A., Peterson, C.M., Tapscott, S.J., Carrell, D.T., Cairns, B.R., 2017. Conserved roles of mouse Dux and human Dux4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet. 49, 925-934. [29] Herceg, Z., Wang, Z.Q., 2001. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat. Res.-Fundam. Mol. Mech. Mutag. 477, 97-110. [30] Hu, W.W., Feng, Z.H., Teresky, A.K., Levine, A.J., 2007. P53 regulates maternal reproduction through lif. Nature 450, 721-U728. [31] Huang, S.M., Mishina, Y.M., Liu, S., Cheung, A., Stegmeier, F., Michaud, G.A., Charlat, O., Wiellette, E., Zhang, Y., Wiessner, S., Hild, M., Shi, X., Wilson, C.J., Mickanin, C., Myer, V., Fazal, A., Tomlinson, R., Serluca, F., Shao, W., Cheng, H., Shultz, M., Rau, C., Schirle, M., Schlegl, J., Ghidelli, S., Fawell, S., Lu, C., Curtis, D., Kirschner, M.W., Lengauer, C., Finan, P.M., Tallarico, J.A., Bouwmeester, T., Porter, J.A., Bauer, A., Cong, F., 2009. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614-620. [32] Huang, Y., Kim, J.K., Do, D.V., Lee, C., Penfold, C.A., Zylicz, J.J., Marioni, J.C., Hackett, J.A., Surani, M.A., 2017. Stella modulates transcriptional and endogenous retrovirus programs during maternal-to-zygotic transition. eLife 6, e22345. [33] Ishiuchi, T., Enriquez-Gasca, R., Mizutani, E., Boskovic, A., Ziegler-Birling, C., Rodriguez-Terrones, D., Wakayama, T., Vaquerizas, J.M., Torres-Padilla, M.E., 2015. Early embryonic-like cells are induced by downregulating replication-dependent chromatin assembly. Nat. Struct. Mol. Biol. 22, 662-671. [34] Johnson, W.H., Loskutoff, N.M., Plante, Y., Betteridge, K.J., 1995. Production of four identical calves by the separation of blastomeres from an in vitro derived four-cell embryo. Vet. Rec. 137, 15-16. [35] Kapoor, A., Yao, W., Ying, H., Hua, S., Liewen, A., Wang, Q., Zhong, Y., Wu, C.J., Sadanandam, A., Hu, B., Chang, Q., Chu, G.C., Al-Khalil, R., Jiang, S., Xia, H., Fletcher-Sananikone, E., Lim, C., Horwitz, G.I., Viale, A., Pettazzoni, P., Sanchez, N., Wang, H., Protopopov, A., Zhang, J., Heffernan, T., Johnson, R.L., Chin, L., Wang, Y.A., Draetta, G., DePinho, R.A., 2014. Yap1 activation enables bypass of oncogenic kras addiction in pancreatic cancer. Cell 158, 185-197. [36] Karimi, M.M., Goyal, P., Maksakova, I.A., Bilenky, M., Leung, D., Tang, J.X., Shinkai, Y., Mager, D.L., Jones, S., Hirst, M., Lorincz, M.C., 2011. DNA methylation and SETDB1/H3k9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell 8, 676-687. [37] Ko, M.S., 2016. Zygotic genome activation revisited: Looking through the expression and function of Zscan4. Curr. top. Dev. Biol. 120, 103-124. [38] Leidenroth, A., Clapp, J., Mitchell, L.M., Coneyworth, D., Dearden, F.L., Iannuzzi, L., Hewitt, J.E., 2012. Evolution of Dux gene macrosatellites in placental mammals. Chromosoma 121, 489-497. [39] Li, R., Zhong, C., Yu, Y., Liu, H., Sakurai, M., Yu, L., Min, Z., Shi, L., Wei, Y., Takahashi, Y., Liao, H.K., Qiao, J., Deng, H., Nunez-Delicado, E., Rodriguez Esteban, C., Wu, J., Izpisua Belmonte, J.C., 2019. Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell 179, 687-702. e618. [40] Macfarlan, T.S., Gifford, W.D., Driscoll, S., Lettieri, K., Rowe, H.M., Bonanomi, D., Firth, A., Singer, O., Trono, D., Pfaff, S.L., 2012. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487, 57-63. [41] Maksakova, I.A., Thompson, P.J., Goyal, P., Jones, S.J., Singh, P.B., Karimi, M.M., Lorincz, M.C., 2013. Distinct roles of KAP1, HP1 and G9a/GLP in silencing of the two-cell-specific retrotransposon MERVL in mouse ES cells. Epigenet. Chromatin 6, 15. [42] Marks, H., Kalkan, T., Menafra, R., Denissov, S., Jones, K., Hofemeister, H., Nichols, J., Kranz, A., Stewart, A.F., Smith, A., Stunnenberg, H.G., 2012. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149, 590-604. [43] Martello, G., Sugimoto, T., Diamanti, E., Joshi, A., Hannah, R., Ohtsuka, S., Gottgens, B., Niwa, H., Smith, A., 2012. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 11, 491-504. [44] Matsui, T., Leung, D., Miyashita, H., Maksakova, I.A., Miyachi, H., Kimura, H., Tachibana, M., Lorincz, M.C., Shinkai, Y., 2010. Proviral silencing in embryonic stem cells requires the histone methyltransferase eset. Nature 464, 927-931. [45] Mitalipov, S.M., Yeoman, R.R., Kuo, H.C., Wolf, D.P., 2002. Monozygotic twinning in rhesus monkeys by manipulation of in vitro-derived embryos. Biol. Reprod. 66, 1449-1455. [46] Moore, N.W., Adams, C.E., Rowson, L.E., 1968. Developmental potential of single blastomeres of the rabbit egg. J. Reprod. Fertil. 17, 527-531. [47] Morgani, S.M., Canham, M.A., Nichols, J., Sharov, A.A., Migueles, R.P., Ko, M.S., Brickman, J.M., 2013. Totipotent embryonic stem cells arise in ground-state culture conditions. Cell Rep. 3, 1945-1957. [48] Nakai-Futatsugi, Y., Niwa, H., 2016. Zscan4 is activated after telomere shortening in mouse embryonic stem cells. Stem Cell Rep. 6, 483-495. [49] Pearson, G., Robinson, F., Beers Gibson, T., Xu, B.E., Karandikar, M., Berman, K., Cobb, M.H., 2001. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 22, 153-183. [50] Pfaff, O., Hildebrandt, C., Waelbroeck, M., Hou, X., Moser, U., Mutschler, E., Lambrecht, G., 1995. The (s)-(+)-enantiomer of dimethindene: A novel M2-selective muscarinic receptor antagonist. Eur. J. Pharmacol. 286, 229-240. [51] Piotrowska-Nitsche, K., Perea-Gomez, A., Haraguchi, S., Zernicka-Goetz, M., 2005. Four-cell stage mouse blastomeres have different developmental properties. Development 132, 479-490. [52] Plachta, N., Bollenbach, T., Pease, S., Fraser, S.E., Pantazis, P., 2011. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat. Cell Biol. 13, 117-123. [53] Rodriguez-Terrones, D., Gaume, X., Ishiuchi, T., Weiss, A., Kopp, A., Kruse, K., Penning, A., Vaquerizas, J.M., Brino, L., Torres-Padilla, M.E., 2018. A molecular roadmap for the emergence of early-embryonic-like cells in culture. Nat. Genet. 50, 106-119. [54] Rossant, J., 1976. Postimplantation development of blastomeres isolated from 4- and 8-cell mouse eggs. J. Embryol. Exp. Morphol. 36, 283-290. [55] Rossant, J., Tam, P.P., 2009. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701-713. [56] Shao, D.D., Xue, W., Krall, E.B., Bhutkar, A., Piccioni, F., Wang, X., Schinzel, A.C., Sood, S., Rosenbluh, J., Kim, J.W., Zwang, Y., Roberts, T.M., Root, D.E., Jacks, T., Hahn, W.C., 2014. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158, 171-184. [57] Shi, J.C., Chen, Q., Li, X., Zheng, X.D., Zhang, Y., Qiao, J., Tang, F.C., Tao, Y., Zhou, Q., Duan, E.K., 2015. Dynamic transcriptional symmetry-breaking in pre-implantation mammalian embryo development revealed by single-cell RNA-seq. Development 142, 3468-3477. [58] Singh, A.M., Hamazaki, T., Hankowski, K.E., Terada, N., 2007. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells 25, 2534-2542. [59] Smith, A.G., 2001. Embryo-derived stem cells: Of mice and men. Annu. Rev. Cell. Dev. Biol. 17, 435-462. [60] Storm, M.P., Kumpfmueller, B., Bone, H.K., Buchholz, M., Ripoll, Y.S., Chaudhuri, J.B., Niwa, H., Tosh, D., Welham, M.J., 2014. Zscan4 is regulated by PI3-kinase and DNA-damaging agents and directly interacts with the transcriptional repressors LSD1 and CtBP2 in mouse embryonic stem cells. Plos One 9, e89821. [61] Tabansky, I., Lenarcic, A., Draft, R.W., Loulier, K., Keskin, D.B., Rosains, J., Rivera-Feliciano, J., Lichtman, J.W., Livet, J., Stern, J.N.H., Sanes, J.R., Eggan, K., 2013. Developmental bias in cleavage-stage mouse blastomeres. Curr. Biol. 23, 21-31. [62] Takahashi, K., Yamanaka, S., 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676. [63] Tarkowski, A.K., 1959. Experiments on the development of isolated blastomers of mouse eggs. Nature 184, 1286-1287. [64] Tian, Q., Wang, X.F., Xie, S.M., Yin, Y., Zhou, L.Q., 2020. H3.3 impedes zygotic transcriptional program activated by Dux. Biochem. Biophys. Res. Commun. 522, 422-427. [65] Torres-Padilla, M.E., Parfitt, D.E., Kouzarides, T., Zernicka-Goetz, M., 2007. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445, 214-218. [66] Wang, W., Li, N., Li, X., Tran, M.K., Han, X., Chen, J., 2015. Tankyrase inhibitors target YAP by stabilizing angiomotin family proteins. Cell Rep. 13, 524-532. [67] Whiddon, J.L., Langford, A.T., Wong, C.J., Zhong, J.W., Tapscott, S.J., 2017. Conservation and innovation in the Dux4-family gene network. Nat. Genet. 49, 935-940. [68] White, M.D., Angiolini, J.F., Alvarez, Y.D., Kaur, G., Zhao, Z.P.W., Mocskos, E., Bruno, L., Bissiere, S., Levi, V., Plachta, N., 2016. Long-lived binding of Sox2 to DNA predicts cell fate in the four-cell mouse embryo. Cell 165, 75-87. [69] Willadsen, S.M., 1981. The developmental capacity of blastomeres from 4-cell and 8-cell sheep embryos. J. Embryol. Exp. Morphol. 65, 165-172. [70] Wilson, M.B., Schreiner, S.J., Choi, H.J., Kamens, J., Smithgall, T.E., 2002. Selective pyrrolo-pyrimidine inhibitors reveal a necessary role for Src family kinases in Bcr-Abl signal transduction and oncogenesis. Oncogene 21, 8075-8088. [71] Xu, Q., Xie, W., 2018. Epigenome in early mammalian development: Inheritance, reprogramming and establishment. Trends Cell Biol. 28, 237-253 [72] Yan, Y.L., Zhang, C., Hao, J., Wang, X.L., Ming, J., Mi, L., Na, J., Hu, X., Wang, Y., 2019. Dppa2/4 and SUMO E3 ligase PIAS4 opposingly regulate zygotic transcriptional program. PLoS Biol. 17, e3000324. [73] Yang, J., Guo, R.P., Wang, H., Ye, X.Y., Zhou, Z.C., Dan, J.M., Wang, H.Y., Gong, P., Deng, W., Yin, Y., Mao, S.Q., Wang, L.B., Ding, J.J., Li, J.S., Keefe, D.L., Dawlaty, M.M., Wang, J.L., Xu, G.L., Liu, L., 2016. Tet enzymes regulate telomere maintenance and chromosomal stability of mouse ESCs. Cell Rep. 15, 1809-1821. [74] Yang, J., Ryan, D.J., Lan, G., Zou, X., Liu, P., 2019. In vitro establishment of expanded-potential stem cells from mouse pre-implantation embryos or embryonic stem cells. Nat. Protoc. 14, 350-378. [75] Yang, J., Ryan, D.J., Wang, W., Tsang, J.C., Lan, G., Masaki, H., Gao, X., Antunes, L., Yu, Y., Zhu, Z., Wang, J., Kolodziejczyk, A.A., Campos, L.S., Wang, C., Yang, F., Zhong, Z., Fu, B., Eckersley-Maslin, M.A., Woods, M., Tanaka, Y., Chen, X., Wilkinson, A.C., Bussell, J., White, J., Ramirez-Solis, R., Reik, W., Gottgens, B., Teichmann, S.A., Tam, P.P.L., Nakauchi, H., Zou, X., Lu, L., Liu, P., 2017a. Establishment of mouse expanded potential stem cells. Nature 550, 393-397. [76] Yang, Y., Liu, B., Xu, J., Wang, J., Wu, J., Shi, C., Xu, Y., Dong, J., Wang, C., Lai, W., Zhu, J., Xiong, L., Zhu, D., Li, X., Yang, W., Yamauchi, T., Sugawara, A., Li, Z., Sun, F., Li, X., Li, C., He, A., Du, Y., Wang, T., Zhao, C., Li, H., Chi, X., Zhang, H., Liu, Y., Li, C., Duo, S., Yin, M., Shen, H., Belmonte, J.C.I., Deng, H., 2017b. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell 169, 243-257. e225. [77] Zalzman, M., Falco, G., Sharova, L.V., Nishiyama, A., Thomas, M., Lee, S.L., Stagg, C.A., Hoang, H.G., Yang, H.T., Indig, F.E., Wersto, R.P., Ko, M.S., 2010. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 464, 858-863. [78] Zhang, Q., Dan, J.M., Wang, H., Guo, R.P., Mao, J., Fu, H.F., Wei, X.W., Liu, L., 2016. Tcstv1 and Tcstv3 elongate telomeres of mouse ES cells. Sci. Rep. 6, 19852 [79] Zhang, W., Chen, F., Chen, R., Xie, D., Yang, J., Zhao, X., Guo, R., Zhang, Y., Shen, Y., Goke, J., Liu, L., Lu, X., 2019. Zscan4c activates endogenous retrovirus MERVL and cleavage embryo genes. Nucleic Acids Res. 47, 8485-8501. 期刊类型引用(11)
1. Jiabei, T., Mok, P.L., Subbiah, S.K. Introduction: Stem cells and their application in research and therapy. Stem Cell Laboratory Techniques: A Guide for Researchers and Students, 2023.  必应学术
必应学术2. Guo, S.-M., Zhang, Y.-R., Ma, B.-X. et al. Regulation of cleavage embryo genes upon DRP1 inhibition in mouse embryonic stem cells. Frontiers in Cell and Developmental Biology, 2023.  必应学术
必应学术3. Li, L., Li, P., Chen, J. et al. Rif1 interacts with non-canonical polycomb repressive complex PRC1.6 to regulate mouse embryonic stem cells fate potential. Cell Regeneration, 2022, 11(1): 25.  必应学术
必应学术4. Olbrich, T., Ruiz, S. Genome architecture and totipotency: An intertwined relation during early embryonic development. BioEssays, 2022, 44(7): 2200029.  必应学术
必应学术5. Li, L., Chen, K., Wu, Y. et al. Epigenome-Metabolome-Epigenome signaling cascade in cell biological processes. Journal of Genetics and Genomics, 2022, 49(4): 279-286.  必应学术
必应学术6. Yang, M., Yu, H., Yu, X. et al. Chemical-induced chromatin remodeling reprograms mouse ESCs to totipotent-like stem cells. Cell Stem Cell, 2022, 29(3): 400-418.e13.  必应学术
必应学术7. Zhang, W., Li, Y., Chen, S. et al. nr0b1 (DAX1) loss of function in zebrafish causes hypothalamic defects via abnormal progenitor proliferation and differentiation. Journal of Genetics and Genomics, 2022, 49(3): 217-229.  必应学术
必应学术8. Uranishi, K., Hirasaki, M., Kitamura, Y. et al. Two DNA binding domains of MGA act in combination to suppress ectopic activation of meiosis-related genes in mouse embryonic stem cells. Stem Cells, 2021, 39(11): 1435-1446.  必应学术
必应学术9. Bagheri-Mohammadi, S.. Protective effects of mesenchymal stem cells on ischemic brain injury: therapeutic perspectives of regenerative medicine. Cell and Tissue Banking, 2021, 22(2): 249-262.  必应学术
必应学术10. Piergentili, R., Del Rio, A., Signore, F. et al. Crispr-cas and its wide-ranging applications: From human genome editing to environmental implications, technical limitations, hazards and bioethical issues. Cells, 2021, 10(5): 969.  必应学术
必应学术11. Sun, K.-Y., Guo, S.-M., Cheng, G.-P. et al. Cleavage-embryo genes and transposable elements are regulated by histone variant h2a.x. Journal of Reproduction and Development, 2021, 67(5): 307-312.  必应学术
必应学术其他类型引用(0)
-





 DownLoad:
DownLoad:
 DownLoad:
DownLoad:

 下载:
下载:

 京公网安备 11010502036328号 京ICP备17033152号
京公网安备 11010502036328号 京ICP备17033152号