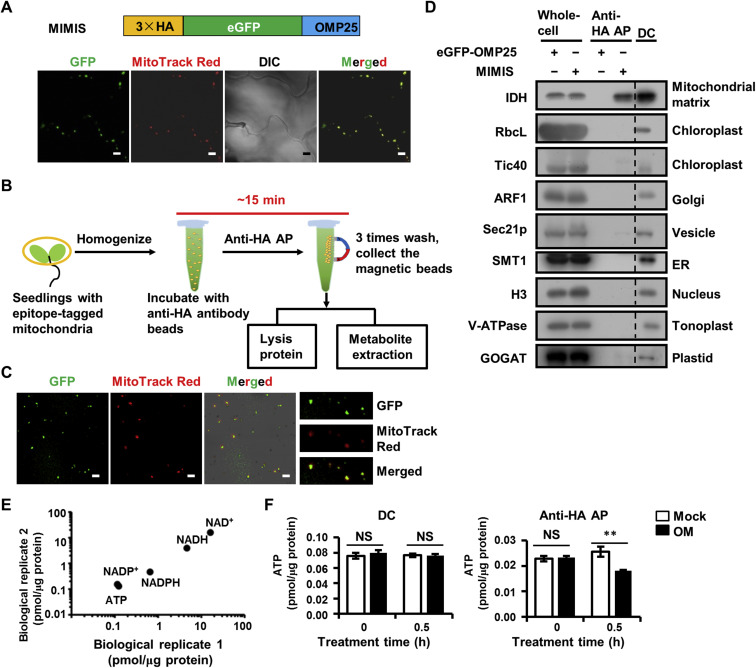
Mitochondria carry out many essential metabolic processes, the dynamic of which impacts most aspects of cellular physiology (Balk and Leaver, 2001; Rose and Sheahan, 2012). Therefore, characterizing the real-time metabolism of mitochondria is of great biological significance. The major challenge for reliable and authentic measurement of mitochondrial metabolites is the pure and rapid isolation of mitochondria, which can avoid the contamination from other organelles and prevent the significant loss of mitochondrial metabolites due to the transmembrane transport and the residual enzymatic reactions during isolation procedure. In plants, the isolation of mitochondria is particularly difficult especially in green leaves (Rao et al., 2017), since the major organelles are chloroplasts and contamination of broken chloroplast fragments is almost inevitable. Up to now, two mitochondria isolation methods, density gradient centrifugation (DC) and free-flow electrophoresis (FFE) by mitochondrial surface charge, have been developed in plants (Millar et al., 2001; Eubel et al., 2007; Lyu et al., 2018). However, these methods have some technical limitations. DC method could not adequately remove other organelles and take 5–6 h to complete, resulting in contamination and a loss of mitochondrial metabolites during isolation procedure. Compared with DC, FFE could largely remove the other organelles but require even longer operation time. Moreover, both DC and FFE require large amount of starting materials for sufficient mitochondria. Recently, an immunopurification method has been developed in mammalian cells, which facilitates the rapid and specific isolation of intact and functional mitochondria and the extraction of mitochondrial metabolites (Chen et al., 2016; Bayraktar et al., 2019; McElroy and Chandel, 2019). Efforts to establish a rapid and effective purification of mitochondria in plant cells were inspired (Kuhnert et al., 2020; Niehaus et al., 2020). Here, we report the affinity purification (AP) of intact and highly purified mitochondria from Arabidopsis leaves within several minutes, emphasizing its advantage in monitoring the rapid metabolic changes of mitochondria in plants.
To achieve rapid and specific isolation of mitochondria in Arabidopsis leaves, the bacterial out mitochondrial membrane protein 25 (OMP25), which is specifically targeted to the outer membrane of mitochondria, is codon optimized for Arabidopsis and adopted as handles for AP. We then fused the mitochondrial localization sequence of OMP25 and coding sequence of 3×HA epitope tag to the C-terminal and N-terminal of enhanced green fluorescent protein (eGFP), respectively, and the resulting 3×HA-eGFP-OMP25 fragment was named as Mitochondria IMmuno-ISolation (MIMIS) (Fig. 1A). The eGFP-OMP25 without HA tag was used as the AP control. The plant binary expression vectors harboring the MIMIS or eGFP-OMP25 fragment were stably transformed into Arabidopsis plants via stable Agrobacterium-mediated transfection. Positive transgenic plants were selected and used for mitochondria isolation. The specific mitochondrial localization of MIMIS protein was confirmed in Arabidopsis leaf cells, as indicated by the complete overlap of the eGFP fluorescent signal with the mitochondrial marker MitoTracker Red staining signal (Fig. 1A). The MIMIS-harboring Arabidopsis leaves were homogenized, and the homogenate supernatant was incubated with the anti-HA antibody-based magnetic beads by gentle rotation at 4 °C to capture the HA-tagged mitochondria. Then, the captured mitochondria were washed for further protein or metabolite analysis. It takes approximately 15 min for the whole isolation process from homogenization, enabling rapid purification of mitochondria from green leaves (Fig. 1B).

The integrity and purity of isolated mitochondria are critical for further metabolite analysis. To test the integrity of the affinity-captured mitochondria, we stained the isolated mitochondria with MitoTracker Red, which can only stain the intact mitochondria. As shown in Fig. 1C, majority of the mitochondria attached to the HA magnetic beads were stained by MitoTracker Red, indicating that most of the captured mitochondria were intact. Subsequently, to investigate the purity of the captured mitochondria, we extracted the protein from the affinity-captured mitochondria or the mitochondria separated by DC method, and performed immunoblot analyses using protein markers for mitochondria and other organelles, including chloroplast, Golgi apparatus, vesicle, endoplasmic reticulum, nucleus, tonoplast and plastid. The immunoblot results showed that mitochondrial matrix marker IDH was detected in the affinity-purified material by the MIMIS but not eGFP-OMP25, and the bands corresponding to the markers of other organelles are almost absent in the MIMIS, indicating the significantly less contamination of other organelles especially the chloroplast in contrast to the DC method (Figs. 1D and S1). Collectively, the rapid affinity-captured mitochondria are intact and highly pure with removal of most other organelles.
To determine the reliability of metabolic analysis using affinity-captured mitochondria, we then quantified important mitochondrial metabolites in Arabidopsis seedlings, including NAD+, NADH, NADP+, NADPH and ATP, by enzymatic determination with total mitochondrial protein normalization (Liang et al., 2015; Luo et al., 2019). Two biological replicates (each with three technical replicates) of all metabolite measurements showed high reproducibility (Fig. 1E). Moreover, the measurement results showed that the total amount of NAD(H) is much more than that of NADP(H) in plant mitochondria. The NAD in mitochondria is mostly oxidized as NADH/NAD+ ratio was 0.25–0.3, but NADP is highly reduced as NADPH/NADP+ ratio was 3.9–4.3. These features are consistent with previous reports (Møller, 2001), indicating the accuracy of metabolic measurement using the affinity-purified mitochondria.
Oligomycin can effectively and specifically inhibit the mitochondrial ATP synthase activity and thus decrease the mitochondrial ATP production (Romanowska et al., 2005). In Arabidopsis seedlings, the cellular ATP/ADP ratio started to decrease after 20-min oligomycin treatment (Geisler et al., 2012). However, the responses of plant mitochondrial metabolites to short-term oligomycin treatment have not been stated, possibly due to the lack of rapid isolation method. Here, we isolated the mitochondria by DC or AP methods from the Arabidopsis seedlings before treatment or treated with oligomycin for 30 min, to measure their mitochondrial matrix ATP contents. In mitochondria isolated by DC, the ATP level after 30 min oligomycin treatment showed no significant changes compared to that of plants before treatment (Fig. 1F), possibly because of the metabolite loss in 5 h isolation. In contrast, in the affinity purified-mitochondria, the significant decrease of the ATP was observed upon 30 min oligomycin treatment. These results indicate that the successful monitoring of in-time mitochondrial metabolite changes depends on the rapid mitochondria purification method ( Fig. 1F and Table S1). Taken together, our results suggest that the AP method for mitochondria isolation can be used to assess the rapid response of mitochondrial metabolism to certain treatment in plant.
Besides the two traditional choices for mitochondria preparation from plant leaves, DC and DC based FFE, now we have an alternative choice of AP method. The AP method surpasses the other two methods in rapid and specific isolation of intact mitochondria and low amount of starting material, which allows accurate metabolite or protein analysis (Table S1). Notably, the DC and FFE cost more than 5 h, whereas the AP method has significantly reduced the overall operation time to a minimum of 15 min, which greatly reduces the loss of mitochondrial metabolites during isolation procedure and expands the application range to measure the rapid or dynamic responses of mitochondrial metabolites. Moreover, the AP method needs minimal 0.2 g plant for mitochondria preparation compared with 5–40 g for the other two approaches (Eubel et al., 2007; Lyu et al., 2018), which greatly expands the application of this method for precious samples, such as profiling the tissue specific mitochondrial metabolites. However, compared with DC and FFE, the AP method requires the transient or stable transgenic material, limiting its application in the plant species which cannot be genetically modified. Furthermore, MIMIS systems with modifications provide the possibility to various applications. By expressingMIMIS driven by certain tissue specific promoter, it could be used to separate the mitochondria from specific tissues. For example, the mitochondria are involved in the cytoplasmic male-sterility and play a critical role in flower development (Carlsson et al., 2008). By using a flower specific promoter, researchers could achieve to separate the mitochondria specifically in flower cells, facilitating the study of mitochondria in flower development. Additionally, as the intact mitochondria could be isolated, this method makes it possible to extract the nuclear acids from the mitochondria to analyze the mitochondrial response at DNA or RNA levels. MIMIS vectors harboring different fluorescent proteins, including YFP, RFP, VENUS, and CFP, and different selective marker genes, including nptII, Bar and hptII, were generated for the easy use of this toolkit (Figs. S2–S5). The basic principle of AP could also be applicable to other organelles. In summary, our study presented a rapid and highly purified mitochondria AP method in plant.
The research was supported by the National Natural Science Foundation of China (Nos. 91854103, 31521001, 31661143025).
| [1] |
Balk, J., Leaver, C., 2001. The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13, 1803-1818.
|
| [2] |
Bayraktar, E, Baudrier, L., Ozerdem, C., Lewis, C.A., Chan, S., Kunchok, T., Abu-Remaileh, M., Cangelosi, A.L., Sabatini, D., Birsoy, K., Chen, W., 2019. MITO-Tag Mice enable rapid isolation and multimodal profiling of mitochondria from specific cell types in vivo. Proc. Natl. Acad. Sci. U. S. A. 116, 303-312.
|
| [3] |
Carlsson, J., Leino, M., Sohlberg, J., Sundstrom, J., Glimelius, K., 2008. Mitochondrial regulation of flower development. Mitochondrion 8, 74-86.
|
| [4] |
Chen, W., Freinkman, E., Wang, T., Birsoy, K., Sabatini, D., 2016. Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell 166, 1324-1337.
|
| [5] |
Eubel, H., Lee, C., Kuo, J., Meyer, E., Taylor, N., Millar, A., 2007. Free-flow electrophoresis for purification of plant mitochondria by surface charge. Plant J. 52, 583-594.
|
| [6] |
Geisler, D., Papke, C., Obata, T., Nunes-Nesi, A., Matthes, A., Schneitz, K., Maximova, E., Araujo, W., Fernie, A., Persson, S., 2012. Downregulation of the δ-Subunit Reduces Mitochondrial ATP Synthase Levels, Alters Respiration, and Restricts Growth and Gametophyte Development in Arabidopsis. Plant Cell 24, 2792.
|
| [7] |
Kuhnert, F., Stefanski A., Overbeck N., Drews L., Reichert A., Stuhler K., Weber A., 2020. Rapid single-step affinity purification of HA-tagged plant mitochondria. Plant Physiol. DOI: 10.1104/pp.19.00732.
|
| [8] |
Liang, C., Zhang, Y., Cheng, S., Osorio, S., Sun, Y., Fernie, A.R., Cheung, C., Lim, B., 2015. Impacts of high ATP supply from chloroplasts and mitochondria on the leaf metabolism of Arabidopsis thaliana. Front. Plant Sci. 6, 922.
|
| [9] |
Luo, L., He, Y., Zhao, Y., Xu, Q., Wu, J., Ma, H., Guo, H., Bai, L., Zuo, J., Zhou, J.-M., Yu, H., Li, J., 2019. Regulation of mitochondrial NAD pool via NAD+ transporter 2 is essential for matrix NADH homeostasis and ROS production in Arabidopsis. Sci. China Life Sci. 62, 991-1002.
|
| [10] |
Millar, A., Sweetlove, L., Giege, P., Leaver, C., 2001. Analysis of the Arabidopsis Mitochondrial Proteome. Plant Physiol. 127, 1711.
|
| [11] |
Moeller, I., 2001. PLANT MITOCHONDRIA AND OXIDATIVE STRESS: Electron Transport, NADPH Turnover, and Metabolism of Reactive Oxygen Species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 561-591.
|
| [12] |
Lyu, W., Selinski, J., Li, L., Day, D., Murcha, M., Whelan, J., Wang, Y., 2018. Isolation and Respiratory Measurements of Mitochondria from Arabidopsis thaliana. JoVE, e56627.
|
| [13] |
McElroy, G., Chandel, N., 2019. Probing mitochondrial metabolism in vivo. Proc. Natl. Acad. Sci. U. S. A. 116, 20-22.
|
| [14] |
Niehaus, M., Straube, H., Kunzler, P., Rugen, N., Hegermann, J. Giavalisco, P., Eubel, H., Witte, C., Herde M., 2020. Rapid affinity purification of tagged plant mitochondria (Mito-AP) for metabolome and proteome analyses. Plant Physiol. DOI: 10.1104/pp.19.00736.
|
| [15] |
Rao, R., Salvato, F., Thal, B., Eubel, H., Thelen, J., Moeller, I., 2017. The proteome of higher plant mitochondria. Mitochondrion 33, 22-37.
|
| [16] |
Romanowska, E., Pokorska, B., Siedlecka, M., 2005. The effects of oligomycin on content of adenylates in mesophyll protoplasts, chloroplasts and mitochondria from Pb2+ treated pea and barley leaves. Acta Physiol. Plant. 27, 29-36.
|
| [17] |
Rose, R. and Sheahan, M., 2012. Plant Mitochondria. In: Encyclopedia of Life Sciences. John Wiley& Sons Ltd, Chichester.
|
| [1] | Xiaojuan Zheng, Zhaoyang Zhou, Zhen Gong, Meijuan Hu, Ye Jin Ahn, Xiaojuan Zhang, Yan Zhao, Guoshu Gong, Jian Zhang, Jianru Zuo, Guan-Zhu Han, Sohn Kee Hoon, Jian-Min Zhou. Two plant NLR proteins confer strain-specific resistance conditioned by an effector from Pseudomonas syringae pv. actinidiae[J]. Journal of Genetics and Genomics, 2022, 49(8): 823-832. doi: 10.1016/j.jgg.2022.06.006 |
| [2] | Jianzhi Zhao, Xiaojie Wang, Xinan Meng, Wei Zou, Suhong Xu. Rapid and efficient wounding for in vivo studies of neuronal dendrite regeneration and degeneration[J]. Journal of Genetics and Genomics, 2021, 48(2): 163-166. doi: 10.1016/j.jgg.2020.10.003 |
| [3] | Mai Yang, Chun Yan, Megan Griffith, Jinping Zhao, Yongbiao Zhang, Daoxin Xie, Jianbin Yan. Arabidopsis EED1 encoding a plant-specific nuclear protein is essential for early embryogenesis[J]. Journal of Genetics and Genomics, 2020, 47(1): 61-64. doi: 10.1016/j.jgg.2019.12.005 |
| [4] | Yidan Ouyang, Qifa Zhang. The molecular and evolutionary basis of reproductive isolation in plants[J]. Journal of Genetics and Genomics, 2018, 45(11): 613-620. doi: 10.1016/j.jgg.2018.10.004 |
| [5] | Guang-Hui Chen, Jia-Ying Sun, Man Liu, Jie Liu, Wei-Cai Yang. SPOROCYTELESS Is a Novel Embryophyte-Specific Transcription Repressor that Interacts with TPL and TCP Proteins in Arabidopsis[J]. Journal of Genetics and Genomics, 2014, 41(12): 617-625. doi: 10.1016/j.jgg.2014.08.009 |
| [6] | Lingling Zhang, Bo Wang, Lei Pan, Junhua Peng. Recycling Isolation of Plant DNA, A Novel Method[J]. Journal of Genetics and Genomics, 2013, 40(1): 45-54. doi: 10.1016/j.jgg.2012.10.001 |
| [7] | Shi-Ming Luo, Heide Schatten, Qing-Yuan Sun. Sperm Mitochondria in Reproduction: Good or Bad and Where Do They Go?[J]. Journal of Genetics and Genomics, 2013, 40(11): 549-556. doi: 10.1016/j.jgg.2013.08.004 |
| [8] | Chuanxian Wei, Jiyong Liu, Zhongsheng Yu, Bo Zhang, Guanjun Gao, Renjie Jiao. TALEN or Cas9 – Rapid, Efficient and Specific Choices for Genome Modifications[J]. Journal of Genetics and Genomics, 2013, 40(6): 281-289. doi: 10.1016/j.jgg.2013.03.013 |
| [9] | Zong-An Huang, Ting Zhao, Hua-Jie Fan, Ning Wang, Shu-Song Zheng, Hong-Qing Ling. The Upregulation of NtAN2 Expression at Low Temperature is Required for Anthocyanin Accumulation in Juvenile Leaves of Lc-transgenic Tobacco (Nicotiana tabacum L.)[J]. Journal of Genetics and Genomics, 2012, 39(3): 149-156. doi: 10.1016/j.jgg.2012.01.007 |
| [10] | Shu-Fen Li, Li-Ying Song, Wei-Bo Yin, Yu-Hong Chen, Liang Chen, Ji-Lin Li, Richard R.-C. Wang, Zan-Min Hu. Isolation and Functional Characterisation of the Genes Encoding Δ8-Sphingolipid Desaturase from Brassica rapa[J]. Journal of Genetics and Genomics, 2012, 39(1): 47-59. doi: 10.1016/j.jgg.2011.12.002 |
| [11] | Young Geol Yoon, Michael Duane Koob. Toward genetic transformation of mitochondria in mammalian cells using a recoded drug-resistant selection marker[J]. Journal of Genetics and Genomics, 2011, 38(4): 173-179. doi: 10.1016/j.jgg.2011.03.005 |
| [12] | Ming Yang, Yan Ge, Jiayan Wu, Jingfa Xiao, Jun Yu. Coevolution study of mitochondria respiratory chain proteins: Toward the understanding of protein–protein interaction[J]. Journal of Genetics and Genomics, 2011, 38(5): 201-207. doi: 10.1016/j.jgg.2011.04.003 |
| [13] | Chuntai Wu, Baoliang Zhou, Tianzhen Zhang. Isolation and characterization of a sterile-dwarf mutant in Asian cotton (Gossypium arboreum L.)[J]. Journal of Genetics and Genomics, 2009, 36(6): 343-353. doi: 10.1016/S1673-8527(08)60123-X |
| [14] | Yanfei Ren, Tao Wang, Yufa Peng, Bin Xia, Li-Jia Qu. Distinguishing transgenic from non-transgenic Arabidopsis plants by 1H NMR-based metabolic fingerprinting[J]. Journal of Genetics and Genomics, 2009, 36(10): 621-628. doi: 10.1016/S1673-8527(08)60154-X |
| [15] | Kun Yuan, Bo Zhang, Yanmei Zhang, Qiang Cheng, Mingxiu Wang, Minren Huang. Identification of differentially expressed proteins in poplar leaves induced by Marssonina brunnea f. sp. Multigermtubi[J]. Journal of Genetics and Genomics, 2008, 35(1): 49-60. doi: 10.1016/S1673-8527(08)60007-7 |
| [16] | Qiang Cheng, Youzhi Cao, Huixin Pan, Mingxiu Wang, Minren Huang. Isolation and characterization of two genes encoding polygalacturonase-inhibiting protein from Populus deltoides[J]. Journal of Genetics and Genomics, 2008, 35(10): 631-638. doi: 10.1016/S1673-8527(08)60084-3 |
| [17] | Yijun Wang, Guangming Yin, Qin Yang, Jihua Tang, Xiaomin Lu, Schuyler S. Korban, Mingliang Xu. Identification and isolation of Mu-flanking fragments from maize[J]. Journal of Genetics and Genomics, 2008, 35(4): 207-213. doi: 10.1016/S1673-8527(08)60029-6 |
| [18] | Wei Sun, Hong Chang, Dejun Ji, Xinjun Liao, Lei Du, Shengxia Lu, Tsunoda Kenji. Analysis on Genetic Diversity and Isolation Mechanism by Distance of Different Ecological Type Sheep Breeds in Mongolia Sheep Group[J]. Journal of Genetics and Genomics, 2007, 34(11): 1001-1009. doi: 10.1016/S1673-8527(07)60113-1 |
| [19] | Changjie Yan, Song Yan, Xiuhong Zeng, Zhengqiu Zhang, Minghong Gu. Fine Mapping and Isolation of Bc7(t), Allelic to OsCesA4[J]. Journal of Genetics and Genomics, 2007, 34(11): 1019-1027. doi: 10.1016/S1673-8527(07)60115-5 |
| [20] | Qiuli Li, Hui Yin, Dan Li, Hongfei Zhu, Yi Zhang, Weiwei Zhu. Isolation and Characterization of CMO Gene Promoter from Halophyte Suaeda liaotungensis K.[J]. Journal of Genetics and Genomics, 2007, 34(4): 355-361. doi: 10.1016/S1673-8527(07)60038-1 |
| 1. | Zu, X., Luo, L., Wang, Z. et al. A mitochondrial pentatricopeptide repeat protein enhances cold tolerance by modulating mitochondrial superoxide in rice. Nature Communications, 2023, 14(1): 6789. doi:10.1038/s41467-023-42269-4 | |
| 2. | Wang, J., Huang, X., Ge, H. et al. The quantitative proteome atlas of a model cyanobacterium. Journal of Genetics and Genomics, 2022, 49(2): 96-108. doi:10.1016/j.jgg.2021.09.007 | |
| 3. | Smith, E.N., Schwarzländer, M., Ratcliffe, R.G. et al. Shining a light on NAD- and NADP-based metabolism in plants. Trends in Plant Science, 2021, 26(10): 1072-1086. doi:10.1016/j.tplants.2021.06.010 | |
| 4. | Luo, L., He, Y., Li, J. et al. Immunopurification of Mitochondria from Arabidopsis. Current Protocols, 2021, 1(2): e34. doi:10.1002/cpz1.34 | |
| 5. | Steinbeck, J., Fuchs, P., Negroni, Y.L. et al. In vivo nadh/nad1 biosensing reveals the dynamics of cytosolic redox metabolism in plants. Plant Cell, 2020, 32(10): 3324-3345. doi:10.1105/tpc.20.00241 | |
| 6. | Lang, M., Pröschel, M., Brüggen, N. et al. Tagging and catching: Rapid isolation and efficient labeling of organelles using the covalent Spy-System in planta. Plant Methods, 2020, 16(1): 122. doi:10.1186/s13007-020-00663-9 |
