Wheat (Triticum aestivum L.) is a world staple food crop, and the improvement of wheat yield is critical for meeting the food demand of the growing global population. Hybrid vigor or heterosis refers to the superior performance of the hybrid progeny over both parents, such as increased yield, enhanced yield stability, faster growth and development, higher biomass, better quality, and enhanced resistance to biotic and abiotic stress (Perez-Prat et al., 2002; Chen et al., 2019). The exploitation of heterosis is considered to be an effective method to improve crop yield and other agronomic traits. In fact, heterosis has been successfully used in various crops including maize and rice (Duvick, 2001; Longin et al., 2012).
As wheat is monoclinous and autogamous (De vries, 1971), the generation, restoration, and maintenance of male sterile lines are the key issues for large-scale commercial hybrid seed production. Since the 1950s, cytoplasmic male sterility (CMS), chemical hybridizing agent (CHA)–induced male sterility, and photoperiod/temperature-sensitive male sterility have been successively exploited for producing hybrid wheat. The first CMS line of wheat was generated by substituting the nuclear genome of common wheat for the original one in Aegilops caudate (Kihara, 1951). Subsequently, more than 70 cytoplasms inducing CMS in wheat have been studied in detail (Singh et al., 2010). The CMS-based three-line hybrid wheat seed production system had been developed (Wilson et al., 1962), but was not widely used commercially. This was due to the limitations of the CMS system, such as high cost of seed production for maintaining the three lines (male sterile line, maintainer, and restorer), unstable or incomplete male sterility (Murai et al., 1993; Liu et al., 1997), limited options for the restoration lines and unreliable fertility restoration (Liu et al., 2002; Geyer et al., 2018), and negative effect of alloplasm and cytoplasm (Tsunewaki et al., 1993). The first CHA-induced male sterility was reported in 1953 (Hoagland et al., 1953). The genotype-independent trait of CHA allowed the exploration of any parental combinations and the production of hybrid wheat cultivar with high-level heterosis (Singh et al., 2010; Whitford et al., 2013). However, highly dependence on growth stage and weather conditions (Pickett, 1993), noncomplete sterility in female, and phytotoxic effects (Adugna et al., 2004) became rather limiting factors. As such, few CHA-induced male sterile lines have been used in commercial hybrid seed production of wheat (Whitford et al., 2013). The two-line hybrid wheat system, based on photoperiod/temperature-sensitive male sterility, does not need maintenance line and is relatively low cost (Zhao, 2013), whereas photoperiod/temperature-sensitive male sterility tends to be influenced by environmental and climate conditions (Chen et al., 2005) and is difficult to be restored completely (Zhang, 2005).
In contrast, a recessive genic male sterile (GMS) mutant has more advantages suitable for the hybrid wheat production. First, it is single recessive nuclear gene controlled and can be restored by any wild-type (WT) germplasms and thus has broader choice of paternal lines to produce hybrid wheat of superior heterosis. Second, it is insensitive to environmental and climate conditions, which reduces the risk caused by weather changes. Third, it avoids the negative alloplasmic and cytoplasmic effects, which is beneficial to the expression of heterosis (Chang et al., 2016; Tucker et al., 2017). The propagation of homozygous male sterile female inbred lines is the only problem for GMS to be used in commercial hybrid production, which has been solved in both maize and rice through seed production technology (SPT) (Chang et al., 2016; Wu et al., 2016; Zhang et al., 2018a). To develop an equivalent system in wheat, identification of nonconditional, nuclear recessive male sterile mutant and its corresponding gene is the first step.
Bread wheat genome is allohexaploid, which consists of three diploid subgenomes (A, B, and D) inherited from three related ancestors (Petersen et al., 2006). Therefore, most of wheat genes have three highly homologous but not identical copies exhibiting extensively functional redundancy, which makes it unusually difficult to obtain the recessive nuclear male sterile mutants through spontaneous mutation or traditional mutagenesis methods. Until now, there are just five stable GMS mutants that have been reported in bread wheat (Pugsleay et al., 1959; Fossati et al., 1970; Driscoll et al., 1977; Sasakuma et al., 1978; Zhou et al., 2008). Among them, only ms1 and ms5 are recessive mutants (Sasakuma et al., 1978; Klindworth et al., 2002).
Recently, the development of genome editing technologies makes it possible to precisely modify the DNA sequences in vivo (Voytas et al., 2014; Vats et al., 2019). Among them, the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein-9 (Cas9) system became the most popular genome editing technology worldwide, because of its specificity, simplicity, flexibility, and versatility (Li et al., 2019). It has been successfully applied in many plant species including model plants (Arabidopsis and Nicotiana benthamiana) (Feng et al., 2013; Nekrasov et al., 2013) and crops (rice, maize, and wheat) (Shan et al., 2013; Wang et al., 2014; Feng et al., 2016).
OsNP1 gene in rice and its maize ortholog ZmIPE1, both of which encode a putative glucose-methanol-choline oxidoreductase, preferentially express in the tapetum and function in tapetum degeneration and pollen exine formation (Chang et al., 2016; Liu et al., 2017; Chen et al., 2017). In this study, we identified three homoeoalleles of OsNP1 in wheat, which show similar expression pattern to OsNP1 and ZmIPE1. To improve the gene editing efficiency in wheat, we optimized the CRISPR/Cas9 vectors in a protoplast assay system. The optimized CRISPR/Cas9 system was used to disrupt endogenous TaNP1 genes and create tanp1 mutants. We found that triple homozygous tanp1 mutant displays complete male sterility and any one WT copy of TaNP1 genes is sufficient for maintenance of male fertility. This work provides an optimized CRISPR/Cas9 vector in wheat genome editing, elucidates the highly conserved biological function of TaNP1 genes, and generated complete male sterile mutants for developing commercial hybrid seed production in wheat.
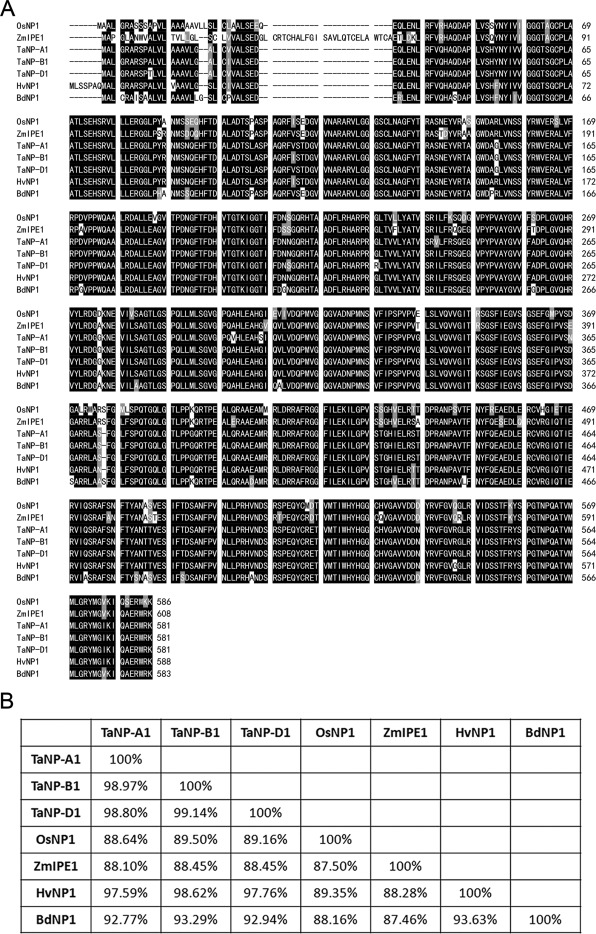
OsNP1 (LOC_Os10g38050) in rice and ZmIPE1 (GRMZM2G434500) in maize are orthologs, which regulate tapetum degeneration and pollen exine formation (Chang et al., 2016; Chen et al., 2017). To explore this gene's function in wheat, three orthologs of OsNP1 were identified by Basic Local Alignment Search Tool (BLAST) analysis in International Wheat Genome Sequencing Consortium (IWGSC) database (http://plants.ensembl.org/Triticum_aestivum/Info/Index) with OsNP1 Coding Sequence (CDS). They are located in chromosome 1A, 1B, and 1D and named as TaNP-A1 (TraesCS1A02G187500), TaNP-B1 (TraesCS1B02G195300), and TaNP-D1 (TraesCS1D02G189200), respectively. All three TaNP1 genes consist of three predicted exons and two introns and encode proteins of 581 amino acids (Fig. 1A). The genomic sequence and CDS of the three genes have been verified in wheat variety CB037. Amino acid sequence alignment showed that the identity between three TaNP1 proteins is 98.8%–99.1%, while the identity of TaNP1s with OsNP1, ZmIPE1, HvNP1 (ortholog of TaNP1 in Hordeum vulgare, HORVU1Hr1G048960.1), and BdNP1 (ortholog of TaNP1 in Brachypodium distachyon, Bradi3g31630.1) is 88.6%–89.5%, 88.1%–88.5%, 97.6%–98.6%, and 92.8%–93.3%, respectively (Fig. 1B).

To study the expression pattern of TaNP1 mRNAs, semi-quantitative RT-PCR and quantitative RT-PCR (qRT-PCR) were performed with specific primers (Table S1). All three TaNP1 genes have similar expression patterns. Their transcripts were only detected in anthers during microspore meiosis and unicellular pollen stage of microgametogenesis (Fig. 2A). Furthermore, RNA in situ hybridization revealed that TaNP1s were strongly expressed in the tapetum at the late stage of meiosis and early unicellular pollen stage, but not expressed in other stages (Fig. 2B). These results were consistent with those of OsNP1 and ZmIPE1 (Chang et al., 2016; Chen et al., 2017), indicating that TaNP1 probably performs similar biological function to that of OsNP1 and ZmIPE1.

To obtain higher editing efficiency in wheat, the CRISPR/Cas9 system was optimized on the basis of the reported system in wheat protoplasts (Shan et al., 2013).
First, we replaced the CaMV 35S promoter with the maize ubiquitin 1 promoter to enhance the expression of Cas9. Target site (TS2), which targets a conserved region in exon 2 of all three TaNP1 genes, was used to assess the editing efficiency.
Second, we compared the activity of four different RNA polymerase III–dependent promoters (Pol III promoter), TaU3p, TaU6p, OsU3p, and OsU6p, which drive the expression of sgRNA (Fig. 3A). Four vectors were transformed into wheat protoplasts, and PCR/Restriction Enzyme (RE) assays were carried out to detect mutations, respectively. Among the four promoters, TaU3 promoter (9.1%–11.3%) appeared to perform much better than the other three (0–4.6%) (Fig. 3A). For each tested promoter, their effects on three TaNP1 gene copies were quite similar in inducing mutation rates. These results suggested that the TaU3 promoter was the best choice of these four Pol III promoters to achieve the highest mutation rate in wheat, and this construct was named as pGR which was used thereafter.

To further optimize the sgRNA cassette in wheat, we compared the mutation rate of the optimized sgRNA scaffold and tRNA-sgRNA system with that of commonly used sgRNA scaffold (Figs. 3B and S1). It was previously reported that the modified sgRNA scaffold with extended duplex length and mutated cytosine at the fourth thymine of the continuous sequence of thymines significantly improved the knockout efficiency in human cells (Chen et al., 2013; Dang et al., 2015). Accordingly, we replaced the original sgRNA scaffold with the optimized one to construct pOPGR vector. Meanwhile in pTRGR vector, a 77-bp-long pre-tRNAGly gene was inserted before TS2-sgRNA scaffold as the endogenous tRNA system has been shown to be a precise and robust tool to produce sgRNAs and achieve higher mutation rate in rice (Xie et al., 2015) (Fig. 3B). The three vectors were transformed into wheat protoplasts, and the mutation rates of three TaNP1s were assessed. pOPGR resulted in the highest mutation rate (19.7%–31.8%), and pGR performed slightly better than pTRGR. Therefore, the optimized sgRNA scaffold driven by TaU3 promoter was the most efficient CRISPR/Cas9 system for wheat and further used in this work.
To disrupt all three TaNP1 genes in vivo and create tanp1-null mutants, six target sites (TS1-TS6) were selected, which are strictly conserved in all three TaNP1 genes, and six corresponding optimized CRISPR/Cas9 vectors (pOPGR-TS1 to pOPGR-TS6) were constructed, respectively. The gene editing efficiency of each vector was assessed in wheat protoplasts. The PCR/RE assay showed that all six vectors, except pOPGR-TS1, could result in mutations in three TaNP1 genes simultaneously. The pOPGR-TS5 led to the highest editing efficiency of 44.4%–48.9%, which is uniform for three TaNP1 genes, followed by pOPGR-TS2, whose editing efficiency varied between 14.0% and 54.2% for three TaNP1 genes (Fig. 3C). Cloning and sequencing of the uncut bands for pOPGR-TS5 and pOPGR-TS2 revealed multiple mutation types in the three TaNP1 genes (Fig. S2). Thus, the two vectors were used in the in planta gene edition of three TaNP1 genes.
We co-transformed the pOPGR-TS5 or pOPGR-TS2 plasmid with pAHC20 (Christensen et al., 1996), which harbors the selectable bar gene, into wheat immature embryos by the particle bombardment method (Yao et al., 2006). With conserved primer sets spanning the target sites, the T0 transgenic plants were identified by PCR/RE assay to detect potential mutations in any TaNP1 genes. For pOPGR-TS5, 21 mutant lines out of 320 T0 transgenic lines (6.56%) were identified, and for pOPGR-TS2, 6 mutant lines out of 103 T0 transgenic lines (5.83%) were identified. Then, specific primers for each gene were designed to detect which TaNP1 gene was mutated. Among 21 T0 mutant lines of pOPGR-TS5, there were 1 line with mutation in TaNP-A1, 1 line with mutation in TaNP-B1, 4 lines with mutations in TaNP-D1, 6 lines with mutations in both TaNP-A1 and TaNP-B1, 2 lines with mutations in both TaNP-A1 and TaNP-D1, and 7 lines with mutations in all three TaNP1 genes (Fig. 4A). Cloning and sequencing of the uncut bands from the above 21 lines confirmed the insertion and/or deletion occurred at TS5 of TaNP1 genes (Fig. 4B). There were 2 lines (T0-2 and T0-8) bearing homozygous or biallelic mutations in all three TaNP1 genes (Fig. 4A and B). Different from pOPGR-TS5, pOPGR-TS2 caused chimeric mutations in all 6 T0 lines (Fig S3A). These data suggested that we have developed an efficient CRISPR/Cas9 system which could edit multiple orthologs simultaneously.

Considering the fact that the transgenic plants might be chimeric and the mutations might be somatic in T0 generation (Lawrenson et al., 2015; Howells et al., 2018), we chose T1 progenies from T0-8 (pOPGR-TS5) for further phenotypic analysis. In T0-8 plants, TS5 sequences were proved to be biallelic in all three TaNP1 genes: for TaNP-A1, there were a 5-bp deletion (named a1) in one copy and a 55-bp insertion (a2) in the other copy, both of which result in frameshift mutations; for TaNP-B1, a 13-bp deletion + 54-bp insertion (b1) leads to a truncated protein, and a 3-bp deletion (b2) only results in 1 amino acid deletion; for TaNP-D1, a 9-bp deletion (d1) results in 3 amino acid deletion, and a 7-bp deletion + 103-bp insertion (d2) causes a truncated protein (Fig. 4B). Thus, the T1 progenies of T0-8 displayed a multitude of genotypes, as shown by genotyping (Table 1). Among the diverse genotypes, we noticed that all plants carrying homozygous b1 set no seeds, while one or two copies of b2 could restore this phenotype, indicating that the 3-bp deletion of b2 in TaNP-B1 did not disrupt its biological function.
| Genotype | Seed set | ||||||
|---|---|---|---|---|---|---|---|
| TaNP-A1 | TaNP-B1 | TaNP-D1 | Plants | Total seeds | Seeds per plant | ||
| a2a2 | b1b1 | d1d1 | 2 | 0 | 0 | ||
| a1a2 | b1b1 | d2d2 | 2 | 0 | 0 | ||
| a2a2 | b1b1 | d1d2 | 1 | 0 | 0 | ||
| a1a2 | b1b1 | d1d2 | 2 | 0 | 0 | ||
| a1a1 | b2b2 | d1d1 | 2 | 252 | 126 | ||
| a1a2 | b2b2 | d1d1 | 2 | 266 | 133 | ||
| a2a2 | b2b2 | d1d2 | 3 | 448 | 149 | ||
| a1a1 | b2b2 | d1d2 | 1 | 27 | 27 | ||
| a1a2 | b1b2 | d1d1 | 3 | 406 | 135 | ||
| a1a2 | b1b2 | d2d2 | 2 | 232 | 116 | ||
| a1a1 | b1b2 | d2d2 | 1 | 38 | 38 | ||
| a2a2 | b1b2 | d2d2 | 1 | 286 | 286 | ||
| a1a2 | b1b2 | d1d2 | 6 | 598 | 100 | ||
| a1a1 | b1b2 | d1d2 | 2 | 107 | 54 | ||
| a2a2 | b1b2 | d1d2 | 2 | 268 | 134 | ||
| WT | WT | WT | 5a | 1130 | 226 | ||
| WT, wild-type. aThese plants are wild-type, which are not the progenies of T0-8. | |||||||
The selected T1 plant carrying biallelic a1 and a2 mutations and homozygous b1 and d2 mutations was pollinated with WT pollens. By genotyping, the triple homozygous mutants were identified in the F2 population and named as tanp1-a1a1b1b1d2d2, which carry homozygous a1, b1, and d1 mutations. Compared with WT plants, tanp1-a1a1b1b1d2d2 mutants have open glumes (Fig. 5A), have smaller and indehiscent anthers without pollen grains (Fig. 5B–D), and set no seeds (Fig. 5E), displaying complete male sterility. There was no obvious difference in vegetative and other reproductive organ development between the WT and triple mutant (Fig. 5B and C). Furthermore, 3138 panicles of tanp1-a1a1b1b1d2d2 triple mutants were bagged, and no one seed was harvested.

The T1 progenies of T0-4 (pOPGR-TS2) were also identified by PCR/RE, and 53 of 118 T1 plants carried mutation in TaNP-A1, TaNP-B1, or TaNP-D. T1 plant 4–28 with heterozygous 1-bp insertions in both TaNP-A1 (a3) and TaNP-D1 (d3) was crossed with T1 plant 4–38 with heterozygous 1-bp deletion in TaNP-B1 (b3) (Fig. S3B and C). In the F2 population, the triple-recessive homozygous mutant plants (tanp1-a3a3b3b3d3d3) were also identified and displayed complete male sterility (Fig. S4). These observations demonstrated that, consistent with their orthologs in rice and maize, TaNP1 genes play an important role in pollen development of wheat.
In F2 population of T0-8 (pOPGR-TS5) or T0-5 (pOPGR-TS2), single homozygous mutants (tanp1-a1a1, b1b1, d2d2, or a3a3, b3b3, d3d3) and double homozygous mutants (tanp1-a1a1b1b1, a1a1d2d2, b1b1d2d2, or a3a3b3b3, a3a3d3d3, b3b3d3d3) were isolated and all of them were fertile with normal anther and microspore development (Figs. 5 and S4), indicating that the three TaNP1 genes are functionally redundant. Meanwhile, the double homozygous-single heterozygous mutants (tanp1-a1a1b1b1Dd2, a1a1Bb1d2d2, Aa1b1b1d2d2 or a3a3b3b3Dd3, a3a3Bb3d3d3, Aa3b3b3d3d3) were also identified and exhibited normal fertility just like WT (Figs. 5 and S4), indicating that although there are 6 copies in wheat, any one copy from three of the TaNP1 genes could perform its biological function to maintain male fertility.
Gene editing technologies, such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR/Cas9 system are becoming powerful tools for crop improvement and breeding. Among them, CRISPR/Cas9 has been extensively used in many plant species because of the multiple advantages, such as simplicity, high efficiency, flexibility, and multiplex capabilities, compared with ZFNs and TALENs. In view of the complexity and big size of wheat genome and the relatively low transformation efficiency, it is quite necessary to further improve the gene editing efficiency of CRISPR/Cas9 in wheat. Because there are two components in the CRISPR/Cas9 system, Cas9 protein and single-guide RNA (sgRNA), which guides Cas9 protein to recognize and cleave target DNA, enhancing expression level of sgRNA andCas9 was conducive to higher gene editing efficiency. Among various Pol III promoters which drive the expression of sgRNA, TaU6 promoter was generally used in the CRISPR/Cas9 system for wheat gene modification (Shan et al., 2013; Zhang et al., 2017, 2019; Bhowmik et al., 2018; Arndell et al., 2019); meanwhile, TaU3 promoter was also occasionally used (Howells et al., 2018; Zhang et al., 2018b). Here, we tested the mutation efficiency of CRISPR/Cas9 vectors with different Pol III promoters (TaU3, TaU6, OsU3, and OsU6 promoters) in wheat protoplasts and found that the vector with TaU3 promoter achieved much higher mutation efficiency (9.1%–11.3%) than other vectors (3.0%–4.5% for TaU6 promoter; 3.0%–4.6% for OsU6 promoter; 0–0.3% for OsU3 promoter). In addition, it has been reported that compared with the commonly used sgRNA, which has a shortened duplex than the native guide RNA and a pause signal for RNA polymerase III (continuous sequence of Ts), the sgRNA with the extended duplex or/and a mutated continuous sequence of Ts (optimized sgRNA) has higher expression level and therefore edits the target genes more efficiently in human cells (Chen et al., 2013; Dang et al., 2015). Other study indicated that using tRNA-sgRNA expression system could enhance the transcript levels of sgRNA and obtain higher mutation rate than traditional sgRNA expression system in rice (Xie et al., 2015). We compared the editing efficiency of different CRISPR/Cas9 vectors with commonly used sgRNA, optimized sgRNA, or tRNAGly-gRNA in wheat protoplast and observed that the optimized sgRNA (19.7%–31.8%) performed better than the commonly used sgRNA (7.7%–12.1%), while tRNAGly-sgRNA (4.9%–8.2%) gave the lowest efficiency. This result is also supported by a recent report, in which optimized sgRNA exhibited higher frequency of single A-to-G conversion than tRNAGly-sgRNA and commonly used sgRNA in both rice and wheat (Li et al., 2018).
OsNP1 gene was first reported in rice, which encodes a putative glucose-methanol-choline oxidoreductase. osnp1 mutant has defects in tapetum degeneration and pollen exine formation, displaying complete male sterility (Chang et al., 2016; Liu et al., 2017). Soon after, ZmIPE1 gene, the ortholog of OsNP1 in maize, was isolated and proved to function in the formation of the anther cuticle, Ubisch bodies, and pollen exine. The anthers of zmipe1 mutant are smaller and withered without mature pollen grains (Chen et al., 2017). We screened the IWGSC database and found there are only three TaNP1 homoeoalleles in wheat genome, which showed high-level homology with each other. The tanp1 triple mutant was generated by CRIPSR/Cas9 and produced no pollen, displaying complete male sterility, similar toosnp1 and zmipe1. This indicates that TaNP1 proteins are functionally conserved in grass and play a similar role in male gametophyte development as their orthologs in rice and maize.
Apparently, the application of the recessive genic male sterility in hybrid seed production showed greater advantages than that of CMS, CHA-induced male sterility, and photoperiod/temperature-sensitive male sterility, such as wide sources of restoring gene, insensitivity to environmental and climate conditions, and no negative alloplasmic and cytoplasmic effect. Until now, only four recessive GMS mutants (tams1, tams5, tams26, and tams45) and their corresponding genes in wheat have been reported. tams1 and tams5 mutants were derived from spontaneous mutation or traditional mutagenesis (Fossati et al., 1970; Driscoll et al., 1975; Franckowiak et al., 1976). They have narrow indehiscent anthers and bear aborted pollen grains. Recently, TaMs1 and TaMs5 have been isolated, both of which encode non-specific lipid transfer proteins (Tucker et al., 2017; Wang et al., 2017; Pallotta et al., 2019). TaMs1 is located in chromosome 4B, whose orthologs in chromosome 4A and 4D are epigenetically silenced during the evolution of allohexaploid wheat (Wang et al., 2017). TaMs5 is located in chromosome 3A (Pallotta et al., 2019). Its ortholog in chromosome 3B (TaMs5-B) contains a deletion and is nonfunctional, while its ortholog in chromosome 3D (TaMs5-D) has two allelic form, nonfunctional and functional ( Pallotta et al., 2019). For this reason, tams5 mutant exhibits male sterility only in the cultivars with nonfunctional TaMs5-D. There are three TaMs26 and TaMs45 homoeoalleles in wheat genome, respectively. The former is the ortholog of maize Ms26 and encodes a cytochrome P450 monooxygenase enzyme (CYP704B1), and the latter is the ortholog of maize Ms45 and encodes a trictosidine synthase–like enzyme. The triple mutant tams26 was achieved through conventional crossing of the single recessive mutants, which were created by a custom-designed homing endonuclease (Cigan et al., 2017; Singh et al., 2017). The triple mutant tams45 was generated by gene edition via CRISPR/Cas9 (Singh et al., 2018). Both tams26 and tams45 produce only a few vestigial microspores in the anthers at the vacuolate stage of microspore development and set few seeds (‘possibly caused by residual pollination’ explained by the authors) (Singh et al., 2017, 2018). In this study, tanp1 triple mutants were created by CRISPR/Cas9 and produce no pollen. Moreover, no single seed was obtained from 3138 bagged panicles of tanp1 triple mutants, showing that tanp1 triple mutants are complete male sterile and suitable for utilization in SPT. Normal male fertility of three double homozygous-single heterozygous mutants (tanp1-aabbDd, tanp1-aaBbdd, and tanp1-Aabbdd) indicates that any one WT copy of TaNP1 genes is sufficient for maintenance of male fertility. Therefore, our work provides a new set of recessive male sterile mutants and its corresponding gene for the development of large-scale commercial hybrid wheat breeding and hybrid seed production.
Wheat variety CB037 was used in this study. All plants were grown in a greenhouse at a white light intensity of 250 mmol/m2s under long-day conditions (16 h of light at 22–25 °C/8 h of dark at 15–20 °C).
Total RNA was isolated using TRI Reagent (Takara Bio Inc., Japan), genomic DNA was removed with DNase I (Promega, Wisconsin, USA), and cDNA was synthetized using a First-Strand cDNA Synthesis Kit (Thermo Fisher, Cleveland, OH, USA). RT-PCR was performed using LA Taq (Takara Bio Inc.). qRT-PCR was performed on a cycler apparatus (Thermo Fisher) using SYBR Premix Ex Taq GC (Takara Bio Inc.) in accordance with the manufacturer's instructions. For qRT-PCR, three technical replicates and three biological replicates were done on each tissue sample, and the amplification data were used to estimate the transcript abundance of TaNP1 genes relative to the transcript abundance of five reference genes (Vandesompele et al., 2002; Burton et al., 2004). The primers used for RT-PCR and qRT-PCR are provided in Table S1.
In situ hybridization was performed according to Wang et al. (2017). Tissues were cut into 8-μm-thick sections, and hybridization was performed overnight at 50 °C. A 924-bp TaNP-D1 fragment was amplified using primers TaNP1-ISH-F/R and inserted into pEASY-T1 Simple Cloning Vector (TransGen Biotech, China) in both forward and reverse orientations. The vectors were linearized by digestion with HindIII or EcoRI and used as a template to generate antisense or sense probes with T7 RNA polymerase.
Rice codon-opitimized Cas9 gene was synthesized and inserted into plasmid pJIT163 to generate plasmid pJIT163-Cas9 as previously reported (Shan et al., 2013). The ubi promoter was amplified from plasmid pAHC20 and inserted into pJIT163-Cas9 between the KpnI and BamHI sites to replace the CaMV 35S promoter, and plasmid pJIT163-ubi::Cas9 was obtained.
The commonly used sgRNA scaffold was synthesized as described previously (Shan et al., 2013). TaU3 promoter and TaU6 promoter were amplified from wheat genomic DNA; OsU3 and OsU6 promoters were amplified from rice genomic DNA. TaU3p/TaU6p/OsU3p/OsU6p:TS2-sgRNA scaffold was obtained through two rounds of PCR reactions and inserted into pJIT163-ubi::Cas9 at KpnI enzyme site to yield vectors shown in Fig. 3A. tRNAGly gene-TS2-sgRNA scaffold was synthesized as described previously (Xie et al., 2015). The cassette TaU3p:tRNAGly gene-TS2-sgRNA scaffold was obtained through two rounds of PCR reactions and inserted into pJIT163-ubi::Cas9 at KpnI enzyme site to yield vector pTRGR (Fig. 3B). The optimized sgRNA scaffold was synthesized as described previously (Dang et al., 2015). The cassette TaU3p:target-optimized sgRNA scaffold was obtained through two rounds of PCR reactions and inserted into pJIT163-ubi::Cas9 at the KpnI site to yield plasmid pOPGR-TS1 to pOPGR-TS6 used in Fig. 3B and C.
The primers used for plasmid construction are provided in Table S2.
Wheat protoplast transformation was performed as previously reported (Shan et al., 2014) with some modifications. Briefly, wheat seeds were grown in soil at 25 °C for 7 days with a photoperiod of 16-h light: 8-h dark. The protoplasts were isolated from the fresh leaves of wheat seedlings, and the plasmids (15 μg) were delivered into protoplasts by Polyethyleneglycol 4000-mediated transfection. After 72 h, the protoplasts were collected to extract DNA for PCR/RE assay.
Biolistic-mediated transformation was performed according to Yao et al. (2006) with minor modifications. Immature embryos, 1.0–1.5 mm in length, were isolated from seeds about 14–16 days after anthesis and precultured for 5–7 days and then used for particle bombardment. Particle bombardment was performed using a PDS1000/He particle bombardment system (Bio-Rad) at helium pressure of 1100 psi. After that, the immature embryos went through resting culture, selection culture, regeneration, and rooting culture successively, and then the transgenic T0 seedlings were obtained.
Specific primers spanning the target sites were designed and tested for genome specificity by sequencing of PCR products with WT genomic DNA as template. Genomic DNA was extracted from pooled protoplasts or transgenic plants. PCR was performed with high-fidelity DNA polymerase and specific primers spanning the target sites. PCR products were digested with the restriction enzyme that recognizes the WT target sequences. The cleaved and uncleaved products were separated by electrophoresis on agarose gel. Mutation rate (%) was calculated by measuring band intensities using software Image J (National Institutes of Health, Bethesda, Maryland, USA). The uncleaved bands were cut, purified, and subcloned into thepEASY-T1 Simple Cloning Vector (TransGen Biotech), and the mutated sequences were identified by sequencing. CRISPR/Cas9 target sequences and enzyme sites are provided in Table S3. Primers used in PCR/RE assay are provided in Table S4.
Jian Li: Investigation, Visualization, Writing - original draft. Zheng Wang: Investigation, Visualization, Writing - original draft. Guangming He: Funding acquisition, Conceptualization. Ligeng Ma: Conceptualization, Funding acquisition, Project administration, Writing - review & editing. Xing Wang Deng: Conceptualization, Funding acquisition, Project administration, Writing - review & editing.
This work was supported by grants from the Ministry of Agriculture of China (2016ZX08010001 and 2016ZX08010002), Peking University Institute of Advanced Agricultural Sciences, and Beijing Natural Science Foundation (19530290014).
| [1] |
Adugna, A., Nanda, G.S., Singh, K., Bains, N.S., 2004. A comparison of cytoplasmic and chemically-induced male sterility systems for hybrid seed production in wheat (Triticum aestivum L.). Euphytica 135, 297-304.
|
| [2] |
Arndell, T., Sharma, N., Langridge, P., Baumann, U., Watson-Haigh, N.S., Whitford, R., 2019. gRNA validation for wheat genome editing with the CRISPR-Cas9 system. BMC Biotechnol. 19, 71-82.
|
| [3] |
Bhowmik, P., Ellison, E., Polley, B., Bollina, V., Kulkarni, M., Ghanbarnia, K., Song, H., Gao, C., Voytas, D.F., Kagale, S., 2018. Targeted mutagenesis in wheat microspores using CRISPR/Cas9. Sci. Rep. 8, 6502-6511.
|
| [4] |
Burton, R.A., Shirley, N.J., King, B.J., Harvey, A.J., Fincher, G.B., 2004. The CesA gene family of barley. Quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiol. 134, 224-236.
|
| [5] |
Chang, Z., Chen, Z., Wang, N., Xie, G., Lu, J., Yan, W., Zhou, J., Tang, X., Deng, X.W., 2016. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. U S A. 113, 14145-14150.
|
| [6] |
Chen, B., Gilbert, L.A., Cimini, B.A., Schnitzbauer, J., Zhang, W., Li, G.W., Park, J., Blackburn, E.H., Weissman, J.S., Qi, L.S., Huang, B., 2013. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479-1491.
|
| [7] |
Chen, E., Huang, X., Tian, Z., Wing, R.A., Han, B., 2019. The genomics of Oryza species provides insights into rice domestication and heterosis. Annu Rev Plant Biol. 70, 639-665.
|
| [8] |
Chen, G., Gong, D., Guo, X., Qiu, W., He, Q., 2005. Problems of the hybrid with Chongqing thermo-photo-sensitive male sterility wheat C49S in the plain of Jiang Han. Mailei Zuowu Xuebao 25, 147-148.
|
| [9] |
Chen, X., Zhang, H., Sun, H., Luo, H., Zhao, L., Dong, Z., Yan, S., Zhao, C., Liu, R., Xu, C., Li, S., Chen, H., Jin, W., 2017. IRREGULAR POLLEN EXINE1 is a novel factor in anther cuticle and pollen exine formation. Plant Physiol. 173, 307-325.
|
| [10] |
Christensen, A.H., Quail, P.H., 1996. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 5, 213-218.
|
| [11] |
Cigan, A.M., Singh, M., Benn, G., Feigenbutz, L., Kumar, M., Cho, M.J., Svitashev, S., Young, J., 2017. Targeted mutagenesis of a conserved anther-expressed P450 gene confers male sterility in monocots. Plant Biotechnol. 15, 379-389.
|
| [12] |
Dang, Y., Jia, G., Choi, J., Ma, H., Anaya, E., Ye, C., Shankar, P., Wu, H., 2015. Optimizing sgRNA structure to improve CRISPR-Cas9 knockout efficiency. Genome Biol. 16, 280-289.
|
| [13] |
De Vries, A.P.H., 1971. Flowering biology of wheat, particularly in view of hybrid seed production - a review. Euphytica 20, 152-170.
|
| [14] |
Duvick, D.N., 2001. Biotechnology in the 1930s: the development of hybrid maize. Nat. Rev. Genet. 2, 69-74.
|
| [15] |
Driscoll, C.J., 1975. Cytogenetic analysis of two chromosomal male-sterility mutants in hexaploid wheat. Aust. J. Biol. Sci. 28, 413-416.
|
| [16] |
Driscoll, C.J., 1977. Registration of Cornerstone male-sterile wheat germplasm. Crop Sci. 17, 190.
|
| [17] |
Feng, Z., Zhang, B., Ding, W., Liu, X., Yang, D.L., Wei, P., Cao, F., Zhu, S., Zhang, F., Mao, Y., Zhu, J.K., 2013. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 23, 1229-1232.
|
| [18] |
Feng, C., Yuan, J., Wang, R., Liu, Y., Birchler, J.A., Han, F., 2016. Efficient targeted genome modification in maize using CRISPR/Cas9 system. J. Genet. Genomics 43, 37-43.
|
| [19] |
Fossati, A., Ingold, M., 1970. A male sterile mutant in Triticum aestivum. Wheat Inform. Serv. 30, 8-10.
|
| [20] |
Franckowiak, J.D., Maan, S.S., Williams, N.D., 1976. A proposal for hybrid wheat utilizing Aegilops squarrosa L. cytoplasm. Crop Science 16, 725-728.
|
| [21] |
Geyer, M., Albrecht, T., Hartl, L., Mohlar, V., 2018. Exploring the genetics of fertility restoration controlled by Rf1 in common wheat (Triticum aestivum L.) using high-density linkage maps. Mol Genet. Genomics 293, 451-462.
|
| [22] |
Hoagland, A.R., Elliott, F.C., Rasmussen, L.W., 1953. Some histological and morphological effects of maleic hydrazide on spring wheat. Agron. J 45, 468-472.
|
| [23] |
Howells, R.M., Craze, M., Bowden, S., Wallington, E.J., 2018. Efficient generation of stable, heritable gene edits in wheat using CRISPR/Cas9. BMC Plant Biol. 18, 215-225.
|
| [24] |
Kihara, H., 1951. Substitution of nucleus and its effects on genome manifestations. Cytologia 16, 177-193.
|
| [25] |
Klindworth, D.L., Williams, N.D., Maan, S.S., 2002. Chromosomal location of genetic male sterility genes in four mutants of hexaploid wheat. Crop Sci. 42, 1447-1450.
|
| [26] |
Lawrenson, T., Shorinola, O., Stacey, N., Li, C., OEstergaard, L., Patron, N., Uauy, C., Harwood, W., 2015. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol. 16, 258-270.
|
| [27] |
Li, C., Zong, Y., Wang, Y., Jin, S., Zhang, D., Song, Q., Zhang, R., Gao, C., 2018. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biol. 19, 59-67.
|
| [28] |
Li, J., Li, Y., Ma, L., 2019. CRISPR/Cas9-Based Genome Editing and its Applications for Functional Genomic Analyses in Plants. Small Methods 3, 1800473-1800493.
|
| [29] |
Liu, C.G., Wu, Y.W., Zhang, C.L., Ren, S.X., Zhang, Y., 1997. A preliminary study on the effects of Aegilops crassa 6x cytoplasm on the characters of common wheat. J Genet Genomics. 24, 241-247.
|
| [30] |
Liu, C.G., Hou, N., Liu, G.Q., Wu, Y.W., Zhang, C.L., Zhang, Y., 2002. Studies on fertility genetic characters in D2-type CMS lines of common wheat. J Genet. Genomics. 29, 638-645.
|
| [31] |
Liu, Z., Lin, S., Shi, J., Yu, J., Zhu, L., Yang, X., Zhang, D., Liang, W., 2017. Rice No Pollen 1 (NP1) is required for anther cuticle formation and pollen exine patterning. Plant J 91, 263-277.
|
| [32] |
Longin, C.F., Muhleisen, J., Maurer, H.P., Zhang, H., Gowda, M., Reif, J.C., 2012. Hybrid breeding in autogamous cereals. Theor. Appl. Genet. 125, 1087-1096.
|
| [33] |
Murai, K., Tsunewaki, K., 1993. Photoperiod-sensitive cytoplasmic male sterility in wheat with Aegilops crassa cytoplasm. Euphytica 67, 41-48.
|
| [34] |
Nekrasov, V., Staskawicz, B., Weigel, D., Jones, J.D., Kamoun, S., 2013. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol. 31, 691-693.
|
| [35] |
Pallotta, M.A., Warner, P., Kouidri, A., Tucker, E.J., Baes, M., Suchecki, R., Watson-Haigh, N., Okada, T., Garcia, M., Sandhu, A., Singh, M., Wolters, P., Albertsen, M.C., Cigan, A.M., Baumann, U., Whitford, R., 2019. Wheat ms5 male-sterility is induced by recessive homoeologous A and D genome non-specific lipid transfer protein. Plant J 99, 673-685.
|
| [36] |
Perez-Prat, E., van Lookeren Campagne, M.M., 2002. Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci. 7, 199-203.
|
| [37] |
Petersen, G., Seberg, O., Yde, M., Berthelsen, K., 2006. Phylogenetic relationships of Triticum and Aegilops and evidence for the origin of the A, B, and D genomes of common wheat (Triticum aestivum). Mol. Phylogenet. Evol. 39, 70-82.
|
| [38] |
Pickett, A.A., 1993. Hybrid wheat: results and problems. Adv. Plant Breeding Suppl. 15, 1-259.
|
| [39] |
Pugsleay, T., Oram, R.N., 1959. Genic male sterility in wheat. Aust. Plant Breed Genet. Newsletter 14, 10-11.
|
| [40] |
Sasakuma, T., Maan, S.S., Williams, N.D., 1978. EMS-induced male-sterile mutants in euplasmic and alloplasmic common wheat. Crop Sci. 18, 850-853.
|
| [41] |
Shan, Q., Wang, Y., Li, J., Zhang, Y., Chen, K., Liang, Z., Zhang, K., Liu, J., Xi, J.J., Qiu, J.L., Gao, C., 2013. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol. 31, 686-688.
|
| [42] |
Shan, Q., Wang, Y., Li, J., Gao, C., 2014. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 9, 2395-2410.
|
| [43] |
Singh, S.K., Chatrath, R., Mishra, B., 2010. Perspective of hybrid wheat research: A review. Indian J Agr. Sci. 80, 1013-1027.
|
| [44] |
Singh, M., Kumar, M., Albertsen, M.C., Young, J.K., Cigan, A.M., 2018. Concurrent modifications in the three homeologs of Ms45 gene with CRISPR-Cas9 lead to rapid generation of male sterile bread wheat (Triticum aestivum L.). Plant Mol. Biol. 97, 371-383.
|
| [45] |
Singh, M., Kumar, M., Thilges, K., Cho, M.J., Cigan, A.M., 2017. MS26/CYP704B is required for anther and pollen wall development in bread wheat (Triticum aestivum L.) and combining mutations in all three homeologs causes male sterility. PLoS One 12, e0177632.
|
| [46] |
Tsunewaki, K., 1993. Genome-plasmon interaction in wheat. Jpn. J Genet. 68, 1-34.
|
| [47] |
Tucker, E.J., Baumann, U., Kouidri, A., Suchecki, R., Baes, M., Garcia, M., Okada, T., Dong, C., Wu, Y., Sandhu, A., Singh, M., Langridge, P., Wolters, P., Albertsen, M.C., Cigan, A.M., Whitford, R., 2017. Molecular identification of the wheat male fertility gene Ms1 and its prospects for hybrid breeding. Nat. Commun. 8, 869-878.
|
| [48] |
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., Speleman, F., 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034.1-11.
|
| [49] |
Vats, S., Kumawat, S., Kumar, V., Patil, G.B., Joshi, T., Sonah, H., Sharma, T.R., Deshmukh, R., 2019. Genome Editing in Plants: Exploration of Technological Advancements and Challenges. Cells 8, 1386-1424.
|
| [50] |
Voytas, D.F., Gao, C., 2014. Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol. 12, e1001877.
|
| [51] |
Wang, Y., Cheng, X., Shan, Q., Zhang, Y., Liu, J., Gao, C., Qiu, J.L., 2014. Simultaneous editing of three homoeoalleles in hexaploid bread confers heritable resistance to powdery mildew. Nat. Biotechnol. 32, 947-951.
|
| [52] |
Wang, Z., Li, J., Chen, S., Heng, Y., Chen, Z., Yang, J., Zhou, K., Pei, J., He, H., Deng, X.W., Ma, L., 2017. Poaceae-specific MS1 encodes a phospholipid-binding protein for male fertility in bread wheat. Proc. Natl. Acad. Sci. USA. 114, 12614-12619.
|
| [53] |
Whitford, R., Fleury, D., Reif, J.C., Garcia, M., Okada, T., Korzun, V., Langridge, P., 2013. Hybrid breeding in wheat: technologies to improve hybrid wheat seed production. J Exp. Bot. 64, 5411-5428.
|
| [54] |
Wilson, J.A., Ross, W.M., 1962. Male sterility interaction of the Triticum aestivum nucleus and Triticum timopheevii cytoplasm. Wheat Inf. Serv. 14, 29-30.
|
| [55] |
Wu, Y., Fox, T.W., Trimnell, M.R., Wang, L., Xu, R.J., Cigan, A.M., Huffman, G.A., Garnaat, C.W., Hershey, H., Albertsen, M.C., 2016. Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnol. J 14, 1046-1054.
|
| [56] |
Xie, K., Minkenberg, B., Yang, Y., 2015. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl. Acad. Sci. USA 112, 3570-3575.
|
| [57] |
Yao, Q., Cong, L., Chang, J.L., Li, K.X., Yang, G.X., He, G.Y., 2006. Low copy number gene transfer and stable expression in a commercial wheat cultivar via particle bombardment. J Exp. Bot. 57, 3737-3746.
|
| [58] |
Zhang, D., Wu, S., An, X., Xie, K., Dong, Z., Zhou, Y., Xu, L., Fang, W., Liu, S., Liu, S., Zhu, T., Li, J., Rao, L., Zhao, J., Wan, X., 2018a. Construction of a multicontrol sterility system for a maize male-sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD-finger transcription factor. Plant Biotechnol. J 16, 459-471.
|
| [59] |
Zhang, F.T., 2005. Fertility transformation characteristics and restoration of photo-thermosensitive genic male sterile wheat. Master degree dissertation of the Chinese Academy of Agricultural Sciences, China.
|
| [60] |
Zhang, S.J., Zhang, R.Z., Song, G.Q., Gao, J., Li, W., Han, X.D., Chen, M.L., Li, Y.L., Li, G.Y., 2018b. Targeted mutagenesis using the Agrobacterium tumefaciens-mediated CRISPR-Cas9 system in common wheat. BMC Plant Biol. 18, 302-313.
|
| [61] |
Zhang, Y., Bai, Y., Wu, G., Zou, S., Chen, Y., Gao, C., Tang, D., 2017. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 91, 714-724.
|
| [62] |
Zhang, Z., Hua, L., Gupta, A., Tricoli, D., Edwards, K.J., Yang, B., Li, W., 2019. Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol. 17, 1623-1635.
|
| [63] |
Zhao, C., 2013. Research and application of hybrid wheat in China. Eng. Sci. 11, 19-21.
|
| [64] |
Zhou, K., Wang, S., Feng, Y., Ji, W., Wang, G., 2008. A new male sterile mutant LZ in wheat (Triticum aestivum L.). Euphytica 159, 403-410.
|
| [1] | Hongning Tong, Chengcai Chu. Coordinating gibberellin and brassinosteroid signaling beyond Green Revolution[J]. Journal of Genetics and Genomics, 2023, 50(7): 459-461. doi: 10.1016/j.jgg.2023.04.009 |
| [2] | Yan Yan, Xiao-Ming Li, Yun Chen, Tian-Tian Wu, Ci-Hang Ding, Mei-Qi Zhang, YueTing Guo, Chu-Yang Wang, Junli Zhang, Xuebin Zhang, Awais Rasheed, Shengchun Xu, Meng-Lu Wang, Zhongfu Ni, Qixin Sun, Jin-Ying Gou. Phosphorylation of KAT-2B by WKS1/Yr36 redirects the lipid flux to jasmonates to enhance resistance against wheat stripe rust[J]. Journal of Genetics and Genomics. doi: 10.1016/j.jgg.2023.08.009 |
| [3] | Ruizhen Yang, Ziyi Yang, Meng Xing, Yexing Jing, Yunwei Zhang, Kewei Zhang, Yun Zhou, Huixian Zhao, Weihua Qiao, Jiaqiang Sun. TaBZR1 enhances wheat salt tolerance via promoting ABA biosynthesis and ROS scavenging[J]. Journal of Genetics and Genomics. doi: 10.1016/j.jgg.2023.09.006 |
| [4] | Libin Chen, Chonghui Ji, Degui Zhou, Xin Gou, Jianian Tang, Yongjie Jiang, Jingluan Han, Yao-Guang Liu, Letian Chen, Yongyao Xie. OsLTP47 may function in a lipid transfer relay essential for pollen wall development in rice[J]. Journal of Genetics and Genomics, 2022, 49(5): 481-491. doi: 10.1016/j.jgg.2022.03.003 |
| [5] | Siyu Chen, Zhiquan Liu, Hao Yu, Liangxue Lai, Zhanjun Li. Efficient multinucleotide deletions using deaminase-Cas9 fusions in human cells[J]. Journal of Genetics and Genomics, 2022, 49(10): 927-933. doi: 10.1016/j.jgg.2022.03.007 |
| [6] | Lin Zhu, Xiaoyan Yang, Juyi Li, Xiong Jia, Xiangli Bai, Ying Zhao, Wenzhuo Cheng, Meng Shu, Yan Zhu, Si Jin. Leptin gene-targeted editing in ob/ob mouse adipose tissue based on the CRISPR/Cas9 system[J]. Journal of Genetics and Genomics, 2021, 48(2): 134-146. doi: 10.1016/j.jgg.2021.01.008 |
| [7] | Jinfu Zhang, Emmanuel M. Khazalwa, Hussein M. Abkallo, Yuan Zhou, Xiongwei Nie, Jinxue Ruan, Changzhi Zhao, Jieru Wang, Jing Xu, Xinyun Li, Shuhong Zhao, Erwei Zuo, Lucilla Steinaa, Shengsong Xie. The advancements, challenges, and future implications of the CRISPR/Cas9 system in swine research[J]. Journal of Genetics and Genomics, 2021, 48(5): 347-360. doi: 10.1016/j.jgg.2021.03.015 |
| [8] | Yan Li, Wenjing Li, Jun Li. The CRISPR/Cas9 revolution continues: From base editing to prime editing in plant science[J]. Journal of Genetics and Genomics, 2021, 48(8): 661-670. doi: 10.1016/j.jgg.2021.05.001 |
| [9] | Fenghua Zhang, Xianmei Li, Mudan He, Ding Ye, Feng Xiong, Golpour Amin, Zuoyan Zhu, Yonghua Sun. Efficient generation of zebrafish maternal-zygotic mutants through transplantation of ectopically induced and Cas9/gRNA targeted primordial germ cells[J]. Journal of Genetics and Genomics, 2020, 47(1): 37-47. doi: 10.1016/j.jgg.2019.12.004 |
| [10] | Chang Ye, Zhuoxin Chen, Zhan Liu, Feng Wang, Xionglei He. Defining endogenous barcoding sites for CRISPR/Cas9-based cell lineage tracing in zebrafish[J]. Journal of Genetics and Genomics, 2020, 47(2): 85-91. doi: 10.1016/j.jgg.2019.11.012 |
| [11] | Xiaohu Su, Wei Chen, Qingqing Cai, Puping Liang, Yaosheng Chen, Peiqing Cong, Junjiu Huang. Production of non-mosaic genome edited porcine embryos by injection of CRISPR/Cas9 into germinal vesicle oocytes[J]. Journal of Genetics and Genomics, 2019, 46(7): 335-342. doi: 10.1016/j.jgg.2019.07.002 |
| [12] | Yufeng Hua, Chun Wang, Jian Huang, Kejian Wang. A simple and efficient method for CRISPR/Cas9-induced mutant screening[J]. Journal of Genetics and Genomics, 2017, 44(4): 207-213. doi: 10.1016/j.jgg.2017.03.005 |
| [13] | Chao Feng, Jing Yuan, Rui Wang, Yang Liu, James A. Birchler, Fangpu Han. Efficient Targeted Genome Modification in Maize Using CRISPR/Cas9 System[J]. Journal of Genetics and Genomics, 2016, 43(1): 37-43. doi: 10.1016/j.jgg.2015.10.002 |
| [14] | Aurélie Lemoine, Gaëlle Chauveau-Le Friec, Francina Langa, Cédric Louvet. Generation of a Double KO Mouse by Simultaneous Targeting of the Neighboring Genes Tmem176a and Tmem176b Using CRISPR/Cas9: Key Steps from Design to Genotyping[J]. Journal of Genetics and Genomics, 2016, 43(5): 329-340. doi: 10.1016/j.jgg.2016.04.004 |
| [15] | Yue Mei, Yan Wang, Huiqian Chen, Zhong Sheng Sun, Xing-Da Ju. Recent Progress in CRISPR/Cas9 Technology[J]. Journal of Genetics and Genomics, 2016, 43(2): 63-75. doi: 10.1016/j.jgg.2016.01.001 |
| [16] | Yuan Lu, Liping Xing, Shujuan Xing, Ping Hu, Chaofan Cui, Mingyi Zhang, Jin Xiao, Haiyan Wang, Ruiqi Zhang, Xiue Wang, Peidu Chen, Aizhong Cao. Characterization of a Putative New Semi-Dominant Reduced Height Gene, Rht_NM9, in Wheat (Triticum aestivum L.)[J]. Journal of Genetics and Genomics, 2015, 42(12): 685-698. doi: 10.1016/j.jgg.2015.08.007 |
| [17] | Chuanxian Wei, Jiyong Liu, Zhongsheng Yu, Bo Zhang, Guanjun Gao, Renjie Jiao. TALEN or Cas9 – Rapid, Efficient and Specific Choices for Genome Modifications[J]. Journal of Genetics and Genomics, 2013, 40(6): 281-289. doi: 10.1016/j.jgg.2013.03.013 |
| [18] | Wenyi Yan, Shenghai Ye, Qingsheng Jin, Longjun Zeng, Yu Peng, Dawei Yan, Weibing Yang, Donglei Yang, Zuhua He, Yanjun Dong, Xiaoming Zhang. Characterization and mapping of a novel mutant sms1 (senescence and male sterility 1) in rice[J]. Journal of Genetics and Genomics, 2010, 37(1): 47-55. doi: 10.1016/S1673-8527(09)60024-2 |
| [19] | Moshe Feldman, Avraham A. Levy. Genome evolution in allopolyploid wheat—a revolutionary reprogramming followed by gradual changes[J]. Journal of Genetics and Genomics, 2009, 36(9): 511-518. doi: 10.1016/S1673-8527(08)60142-3 |
| [20] | Yi Zhang, Yunfeng Li, Jian Zhang, Fucheng Shen, Yuanxin Huang, Zhiwei Wu. Characterization and mapping of a new male sterility mutant of anther advanced dehiscence (t) in rice[J]. Journal of Genetics and Genomics, 2008, 35(3): 177-182. doi: 10.1016/S1673-8527(08)60024-7 |
| 1. | Shen, X., Dong, Q., Zhao, X. et al. Targeted mutation of BnaMS1/BnaMS2 combined with the RUBY reporter enables an efficient two-line system for hybrid seed production in Brassica napus. Horticulture Research, 2025, 12(1): uhae270. doi:10.1093/hr/uhae270 | |
| 2. | Pan, W., Gao, C., Niu, D. et al. Efficient gene disruption in polyploid genome by Cas9–Trex2 fusion protein. Journal of Integrative Plant Biology, 2025, 67(1): 7-10. doi:10.1111/jipb.13797 | |
| 3. | Gawande, N.D., Bhalla, H., Watts, A. et al. Application of genome editing in plant reproductive biology: recent advances and challenges. Plant Reproduction, 2024, 37(4): 441-462. doi:10.1007/s00497-024-00506-w | |
| 4. | Roychowdhury, R., Ghatak, A., Kumar, M. et al. Accelerating wheat improvement through trait characterization: advances and perspectives. Physiologia Plantarum, 2024, 176(5): e14544. doi:10.1111/ppl.14544 | |
| 5. | Sapara, V., Khisti, M., Yogendra, K. et al. Gene editing tool kit in millets: present status and future directions. Nucleus (India), 2024, 67(1): 157-179. doi:10.1007/s13237-024-00485-3 | |
| 6. | Hwarari, D., Radani, Y., Ke, Y. et al. CRISPR/Cas genome editing in plants: mechanisms, applications, and overcoming bottlenecks. Functional and Integrative Genomics, 2024, 24(2): 50. doi:10.1007/s10142-024-01314-1 | |
| 7. | Farooq, A., Khan, U.M., Khan, M.A. et al. Male sterility systems and their applications in hybrid wheat breeding. Cereal Research Communications, 2024, 52(1): 25-37. doi:10.1007/s42976-023-00376-4 | |
| 8. | Shi, Y.-X., Liu, X.-Y., Sun, J.-Q. et al. Knockout of GmBADH1 gene using CRISPR/Cas9 technique to reduce salt tolerance in soybean | [利用 CRISPR/Cas9 技术编辑 GmBADH1 基因改变大豆耐盐性]. Acta Agronomica Sinica(China), 2024, 50(1): 100-109. doi:10.3724/SP.J.1006.2024.34056 | |
| 9. | Garcia-Oliveira, A.L., Ortiz, R., Sarsu, F. et al. The importance of genotyping within the climate-smart plant breeding value chain – integrative tools for genetic enhancement programs. Frontiers in Plant Science, 2024, 15: 1518123. doi:10.3389/fpls.2024.1518123 | |
| 10. | Godara, M., Das, D., Roy, J. et al. Genetic Engineering Methods for Wheat Improvement. Genetic Engineering of Crop Plants for Food and Health Security: Volume 1, 2024. doi:10.1007/978-981-99-5034-8_21 | |
| 11. | Panda, D., Baig, M.J., Molla, K.A. Genome Editing and Opportunities for Trait Improvement in Pearl Millet. Pearl Millet in the 21st Century: Food-Nutrition-Climate Resilience-Improved Livelihoods, 2024. doi:10.1007/978-981-99-5890-0_7 | |
| 12. | Kale, A.N., Kumar, R., Mane, R.S. et al. Genome Editing Technologies for Accelerating Crop Improvement: An Updated Overview. Plant Speed Breeding and High-Throughput Technologies, 2024. doi:10.1201/b23372-6 | |
| 13. | Niu, D., Gao, Z., Cui, B. et al. A molecular mechanism for embryonic resetting of winter memory and restoration of winter annual growth habit in wheat. Nature Plants, 2024, 10(1): 37-52. doi:10.1038/s41477-023-01596-6 | |
| 14. | Pouramini, P., Hensel, G. Precise gene editing of cereals using CRISPR/Cas technology. A Roadmap for Plant Genome Editing, 2023. doi:10.1007/978-3-031-46150-7_9 | |
| 15. | Cheng, H., Hao, M., Sang, S. et al. Establishment of new convenient two-line system for hybrid production by targeting mutation of OPR3 in allopolyploid Brassica napus. Horticulture Research, 2023, 10(12): uhad218. doi:10.1093/hr/uhad218 | |
| 16. | Ahmar, S., Hensel, G., Gruszka, D. CRISPR/Cas9-mediated genome editing techniques and new breeding strategies in cereals – current status, improvements, and perspectives. Biotechnology Advances, 2023, 69: 108248. doi:10.1016/j.biotechadv.2023.108248 | |
| 17. | Nishiguchi, M., Futamura, N., Endo, M. et al. CRISPR/Cas9-mediated disruption of CjACOS5 confers no-pollen formation on sugi trees (Cryptomeria japonica D. Don). Scientific Reports, 2023, 13(1): 11779. doi:10.1038/s41598-023-38339-8 | |
| 18. | Zhou, X., Zhao, Y., Ni, P. et al. CRISPR-mediated acceleration of wheat improvement: advances and perspectives. Journal of Genetics and Genomics, 2023, 50(11): 815-834. doi:10.1016/j.jgg.2023.09.007 | |
| 19. | Tang, Q., Wang, X., Jin, X. et al. CRISPR/Cas Technology Revolutionizes Crop Breeding. Plants, 2023, 12(17): 3119. doi:10.3390/plants12173119 | |
| 20. | Gautam, R., Shukla, P., Kirti, P.B. Male sterility in plants: an overview of advancements from natural CMS to genetically manipulated systems for hybrid seed production. Theoretical and Applied Genetics, 2023, 136(9): 195. doi:10.1007/s00122-023-04444-5 | |
| 21. | Wang, B., Meng, T., Xiao, B. et al. Fighting wheat powdery mildew: from genes to fields. Theoretical and Applied Genetics, 2023, 136(9): 196. doi:10.1007/s00122-023-04445-4 | |
| 22. | Yigider, E., Taspinar, M.S., Agar, G. Advances in bread wheat production through CRISPR/Cas9 technology: a comprehensive review of quality and other aspects. Planta, 2023, 258(3): 55. doi:10.1007/s00425-023-04199-9 | |
| 23. | Bekalu, Z.E., Panting, M., Bæksted Holme, I. et al. Opportunities and Challenges of In Vitro Tissue Culture Systems in the Era of Crop Genome Editing. International Journal of Molecular Sciences, 2023, 24(15): 11920. doi:10.3390/ijms241511920 | |
| 24. | Bakshi, S., Kumar, U., Bhati, P. et al. Genome editing of wheat with CRISPR/Cas systems: Past present and future. CRISPR/Cas-Mediated Genome Editing in Plants, 2023.  | |
| 25. | May, D., Paldi, K., Altpeter, F. Targeted mutagenesis with sequence-specific nucleases for accelerated improvement of polyploid crops: Progress, challenges, and prospects. Plant Genome, 2023, 16(2): e20298. doi:10.1002/tpg2.20298 | |
| 26. | Zhou, J., Luan, X., Liu, Y. et al. Strategies and Methods for Improving the Efficiency of CRISPR/Cas9 Gene Editing in Plant Molecular Breeding. Plants, 2023, 12(7): 1478. doi:10.3390/plants12071478 | |
| 27. | Choudhary, P., Shukla, P., Muthamilarasan, M. Genetic enhancement of climate-resilient traits in small millets: A review. Heliyon, 2023, 9(4): e14502. doi:10.1016/j.heliyon.2023.e14502 | |
| 28. | Zhang, R., Zhang, S., Li, J. et al. CRISPR/Cas9-targeted mutagenesis of TaDCL4, TaDCL5 and TaRDR6 induces male sterility in common wheat. Plant Biotechnology Journal, 2023, 21(4): 839-853. doi:10.1111/pbi.14000 | |
| 29. | Luo, K., He, D., Guo, J. et al. Molecular Advances in Breeding for Durable Resistance against Pests and Diseases in Wheat: Opportunities and Challenges. Agronomy, 2023, 13(3): 628. doi:10.3390/agronomy13030628 | |
| 30. | Yin, W., Chen, Z., Huang, J. et al. Application of CRISPR-Cas9 gene editing technology in crop breeding | [基于 CRISPR-Cas9 基因编辑技术在作物中的应用]. Shengwu Gongcheng Xuebao/Chinese Journal of Biotechnology, 2023, 39(2): 399-424. doi:10.13345/j.cjb.220664 | |
| 31. | Awan, M.J.A., Anjum, N., Pervaiz, K. et al. Genome Editing: Mechanism and Utilization in Plant Breeding. Advanced Crop Improvement: Volume 1: Theory and Practice, 2023, 1: 379-455. doi:10.1007/978-3-031-28146-4_16 | |
| 32. | Khan, Z., Shahwar, D. Genome editing in plants via CRISPR/Cas9: A genomic scissor borrowed from bacterial immune system. Genome Editing and Global Food Security: Molecular Engineering Technologies for Sustainable Agriculture, 2023. doi:10.4324/9781003382102-1 | |
| 33. | Farinati, S., Draga, S., Betto, A. et al. Current insights and advances into plant male sterility: new precision breeding technology based on genome editing applications. Frontiers in Plant Science, 2023, 14: 1223861. doi:10.3389/fpls.2023.1223861 | |
| 34. | Ahmad, N., Fatima, S., Mehmood, M.A. et al. Targeted genome editing in polyploids: lessons from Brassica. Frontiers in Plant Science, 2023, 14: 1152468. doi:10.3389/fpls.2023.1152468 | |
| 35. | Afroz, N., Ansary, M.W.R., Islam, T. CRISPR-Cas genome editing for the development of abiotic stress-tolerant wheat. Abiotic Stresses in Wheat: Unfolding the Challenges, 2023. doi:10.1016/B978-0-323-95368-9.00014-X | |
| 36. | Lorenzo, C.D., Debray, K., Herwegh, D. et al. BREEDIT: A multiplex genome editing strategy to improve complex quantitative traits in maize. Plant Cell, 2023, 35(1): 218-238. doi:10.1093/plcell/koac243 | |
| 37. | Kuluev, B.R., Mikhailova, E.V., Kuluev, A.R. et al. Genome Editing in Species of the Tribe Triticeae with the CRISPR/Cas System. Molecular Biology, 2022, 56(6): 885-901. doi:10.1134/S0026893322060127 | |
| 38. | Gökdemir, G., Seçgin, Z., Uluisik, S. et al. CRISPR/Cas9 knock-out of SlPHD_MS1 (Solyc04g008420) gene results in complete male sterility in tomato. Plant Growth Regulation, 2022, 98(2): 329-341. doi:10.1007/s10725-022-00869-y | |
| 39. | Awan, M.J.A., Pervaiz, K., Rasheed, A. et al. Genome edited wheat- current advances for the second green revolution. Biotechnology Advances, 2022, 60: 108006. doi:10.1016/j.biotechadv.2022.108006 | |
| 40. | Gohar, S., Sajjad, M., Zulfiqar, S. et al. Domestication of newly evolved hexaploid wheat—A journey of wild grass to cultivated wheat. Frontiers in Genetics, 2022, 13: 1022931. doi:10.3389/fgene.2022.1022931 | |
| 41. | Liu, H., Chen, W., Li, Y. et al. CRISPR/Cas9 Technology and Its Utility for Crop Improvement. International Journal of Molecular Sciences, 2022, 23(18): 10442. doi:10.3390/ijms231810442 | |
| 42. | Dhakate, P., Sehgal, D., Vaishnavi, S. et al. Comprehending the evolution of gene editing platforms for crop trait improvement. Frontiers in Genetics, 2022, 13: 876987. doi:10.3389/fgene.2022.876987 | |
| 43. | Kashtwari, M., Mansoor, S., Wani, A.A. et al. Random mutagenesis in vegetatively propagated crops: opportunities, challenges and genome editing prospects. Molecular Biology Reports, 2022, 49(6): 5729-5749. doi:10.1007/s11033-021-06650-0 | |
| 44. | Rao, Y., Yang, X., Pan, C. et al. Advance of Clustered Regularly Interspaced Short Palindromic Repeats-Cas9 System and Its Application in Crop Improvement. Frontiers in Plant Science, 2022, 13: 839001. doi:10.3389/fpls.2022.839001 | |
| 45. | Liu, X., Zhang, S., Jiang, Y. et al. Use of CRISPR/Cas9-Based Gene Editing to Simultaneously Mutate Multiple Homologous Genes Required for Pollen Development and Male Fertility in Maize. Cells, 2022, 11(3): 439. doi:10.3390/cells11030439 | |
| 46. | Mohan, C., Satish, L., Muthubharathi, B.C. et al. CRISPR-Cas Technology: A Genome-Editing Powerhouse for Molecular Plant Breeding. Biotechnological Innovations for Environmental Bioremediation, 2022. doi:10.1007/978-981-16-9001-3_32 | |
| 47. | Zhu, X., Xu, Y., Li, J. et al. Establishment of heterotic groups for hybrid wheat breeding | [小麦杂种优势群的创制]. Kexue Tongbao/Chinese Science Bulletin, 2022, 67(26): 3152-3164. doi:10.1360/TB-2022-0392 | |
| 48. | Wang, Q., He, Z., Wang, L. et al. Advances in chromosome engineering for hybrid wheat breeding | [染色体工程在杂交小麦育种中的应用进展]. Kexue Tongbao/Chinese Science Bulletin, 2022, 67(26): 3129-3139. doi:10.1360/TB-2022-0356 | |
| 49. | Li, J., Zhou, K., Wang, Z. et al. Research progress, problems, and prospects in hybrid wheat seed production technology based on recessive nuclear genetic male sterile lines | [基于隐性核雄性不育系的杂交小麦制种技术研究进展、问题与展望]. Kexue Tongbao/Chinese Science Bulletin, 2022, 67(26): 3140-3151. doi:10.1360/TB-2022-0386 | |
| 50. | Liu, Q., Qi, J., Wu, J. et al. Progress in identifying male sterility genes and utilizing heterosis in wheat | [小麦雄性不育基因的鉴定和杂种优势利用]. Kexue Tongbao/Chinese Science Bulletin, 2022, 67(26): 3100-3109. doi:10.1360/TB-2022-0430 | |
| 51. | Krishna, T.P.A., Maharajan, T., Ceasar, S.A. Application of CRISPR/Cas9 Genome Editing System to Reduce the Pre-and Post-Harvest Yield Losses in Cereals. Open Biotechnology Journal, 2022, 16(1): e187407072205190. doi:10.2174/18740707-V16-E2205190 | |
| 52. | Li, J., Li, Y., Ma, L. Recent advances in CRISPR/Cas9 and applications for wheat functional genomics and breeding. aBIOTECH, 2021, 2(4): 375-385. doi:10.1007/s42994-021-00042-5 | |
| 53. | Hisano, H., Abe, F., Hoffie, R.E. et al. Targeted genome modifications in cereal crops. Breeding Science, 2021, 71(4): 405-416. doi:10.1270/jsbbs.21019 | |
| 54. | Verma, A.K., Mandal, S., Tiwari, A. et al. Current status and perspectives on the application of crispr/cas9 gene-editing system to develop a low-gluten, non-transgenic wheat variety. Foods, 2021, 10(10): 2351. doi:10.3390/foods10102351 | |
| 55. | Ahmad, S., Tang, L., Shahzad, R. et al. CRISPR-Based Crop Improvements: A Way Forward to Achieve Zero Hunger. Journal of Agricultural and Food Chemistry, 2021, 69(30): 8307-8323. doi:10.1021/acs.jafc.1c02653 | |
| 56. | Singh, M., Albertsen, M.C., Cigan, A.M. Male fertility genes in bread wheat (Triticum aestivum l.) and their utilization for hybrid seed production. International Journal of Molecular Sciences, 2021, 22(15): 8157. doi:10.3390/ijms22158157 | |
| 57. | Schaart, J.G., van de Wiel, C.C.M., Smulders, M.J.M. Genome editing of polyploid crops: prospects, achievements and bottlenecks. Transgenic Research, 2021, 30(4): 337-351. doi:10.1007/s11248-021-00251-0 | |
| 58. | Li, S., Zhang, C., Li, J. et al. Present and future prospects for wheat improvement through genome editing and advanced technologies. Plant Communications, 2021, 2(4): 100211. doi:10.1016/j.xplc.2021.100211 | |
| 59. | Ahmar, S., Mahmood, T., Fiaz, S. et al. Advantage of Nanotechnology-Based Genome Editing System and Its Application in Crop Improvement. Frontiers in Plant Science, 2021, 12: 663849. doi:10.3389/fpls.2021.663849 | |
| 60. | Gao, C.. Genome engineering for crop improvement and future agriculture. Cell, 2021, 184(6): 1621-1635. doi:10.1016/j.cell.2021.01.005 | |
| 61. | Milner, M.J., Craze, M., Hope, M.S. et al. Turning Up the Temperature on CRISPR: Increased Temperature Can Improve the Editing Efficiency of Wheat Using CRISPR/Cas9. Frontiers in Plant Science, 2020, 11: 583374. doi:10.3389/fpls.2020.583374 | |
| 62. | Nagar, S., Moola, A.K., Satish, L. et al. Advances in Genetically Modified Plants by Employing Modern Biotechnological Tools: An Update. Policy Issues in Genetically Modified Crops: A Global Perspective, 2020. doi:10.1016/B978-0-12-820780-2.00022-4 |

| Genotype | Seed set | ||||||
|---|---|---|---|---|---|---|---|
| TaNP-A1 | TaNP-B1 | TaNP-D1 | Plants | Total seeds | Seeds per plant | ||
| a2a2 | b1b1 | d1d1 | 2 | 0 | 0 | ||
| a1a2 | b1b1 | d2d2 | 2 | 0 | 0 | ||
| a2a2 | b1b1 | d1d2 | 1 | 0 | 0 | ||
| a1a2 | b1b1 | d1d2 | 2 | 0 | 0 | ||
| a1a1 | b2b2 | d1d1 | 2 | 252 | 126 | ||
| a1a2 | b2b2 | d1d1 | 2 | 266 | 133 | ||
| a2a2 | b2b2 | d1d2 | 3 | 448 | 149 | ||
| a1a1 | b2b2 | d1d2 | 1 | 27 | 27 | ||
| a1a2 | b1b2 | d1d1 | 3 | 406 | 135 | ||
| a1a2 | b1b2 | d2d2 | 2 | 232 | 116 | ||
| a1a1 | b1b2 | d2d2 | 1 | 38 | 38 | ||
| a2a2 | b1b2 | d2d2 | 1 | 286 | 286 | ||
| a1a2 | b1b2 | d1d2 | 6 | 598 | 100 | ||
| a1a1 | b1b2 | d1d2 | 2 | 107 | 54 | ||
| a2a2 | b1b2 | d1d2 | 2 | 268 | 134 | ||
| WT | WT | WT | 5a | 1130 | 226 | ||
| WT, wild-type. aThese plants are wild-type, which are not the progenies of T0-8. | |||||||